Abstract
Longevity is a highly variable life history trait and its variation is attributable to both genetic and environmental factors. Exploring well-known environmental factors in a new model system is a useful approach to explore taxonomic variation in plasticity of longevity. We examined responsiveness of the Daphnia pulex clone TCO to potentially related interventions that have been reported to extend lifespan: resveratrol and dietary restriction. First, we examined effects of resveratrol on lifespan and fecundity in TCO which were grown at moderate (12K cells Ankistrodesmus falcatus mL−1) and high (20K cells A. falcatus mL−1) food levels. We found no evidence for lifespan extension by resveratrol, but found a reduction of lifetime fecundity. The effect of resveratrol on fecundity was more pronounced early in life. We then conducted an additional life table to test the effect of dietary restriction on TCO. Surprisingly, reduced food level did not extend the lifespan of TCO, which contrasts with previous studies in D. pulex. Our results suggest that variation in the response to dietary restriction might be more common than previously thought. If resveratrol activates genes involved in the response to dietary restriction, genetic polymorphisms in dietary restriction will influence responses to resveratrol. Thus, this experiment suggests that careful re-examination of resveratrol effects using diverse genotypes is required.
Considerable variation in the rate of aging and its associated lifespan exists in nature within species. Both genetic and environmental factors contribute to natural variation, but the heritability of lifespan is often relatively small, such that genetic factors account for <35% of the variance of lifespan within species (Finch and Tanzi, ’97). Thus, characterizing plasticity of lifespan is critical to understanding its variation. In addition, identifying environmental or chemical factors that induce differential patterns of aging is important in aging research since those factors can be a useful tool to explore physiological mechanisms of aging, and to develop practical strategies to promote healthy aging.
Numerous studies have demonstrated that a reduced diet without malnutrition (“dietary restriction”) is a strong intervention that significantly increases lifespan, and genes involved in the dietary restriction response are evolutionarily conserved across a wide range of taxa from yeast to mice (Lee et al., 2006; Bishop and Guarente, 2007; Mair and Dillin, 2008; Narasimhan et al., 2009; Fontana et al., 2010). However, food effects on aging may differ among species, or even among genotypes within species (Partridge et al., 2005; Harper et al., 2006; Mockett et al., 2006; Liao et al., 2010). For instance, two recent studies using rhesus monkeys showed contrasting results, such that one study found dietary restriction induced longer lifespan with slow aging (Colman et al., 2009), but the other study did not (Mattison et al., 2012). Notably, genetic diversity of monkey cohorts used for the experiment was greater in latter study. Mechanisms by which dietary restriction extends lifespan may be more complicated than previous studies suggested.
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a polyphenolic phytoalexin produced by plant species as an anti-microbial defense compound. Resveratrol has effects on diverse cellular processes including cell metabolism, the cell cycle, and apoptosis. As a consequence, it has the potential to alleviate age-related diseases and extend lifespan (Howitz et al., 2003; Wood et al., 2004; Baur et al., 2006; Valenzano et al., 2006; Knutson and Leeuwenburgh, 2008; Chachay et al., 2011). The effects of resveratrol are thought to occur through cellular and organismal alterations similar to those promoted by dietary restriction. In particular, resveratrol is hypothesized to activate sirtuins (NAD+ dependent protein deacetylases), which are key players in dietary restriction (Barger et al., 2008; Pearson et al., 2008).
Recently, however, the effect of resveratrol on lifespan has been called into question. Resveratrol might not activate sirtuins in vivo (Kaeberlein et al., 2005; Pacholec et al., 2010), and sirtuins might not be involved in the extension of lifespan (Burnett et al., 2011). Some researchers have reported that resveratrol increased life span modestly, but the effect was either not or only marginally significant in Caenorhabditis elegans, Drosophila melanogaster, and mice (Bass et al., 2007; Miller et al., 2011), despite the fact that gene expression patterns of animals treated with resveratrol had some degree of similarity to those with low diet (Barger et al., 2008; Pearson et al., 2008). In addition, survival curves of animals treated with resveratrol are sometimes different from those with dietary restriction (Valenzano et al., 2006; Chandrashekara and Shakarad, 2012). Therefore, the physiological mechanisms of resveratrol effect on lifespan might be independent of the mechanisms that dietary restriction has and/or they might be sensitive to genetic background of testing organisms (Kaeberlein et al., 2005; Burnett et al., 2011).
Although life history biologists are well aware of tradeoffs between survival and reproduction, relatively few studies have investigated the effects of resveratrol on reproduction. Gruber et al. (2007) provided some evidence of a tradeoff in C. elegans, showing that resveratrol delayed the distribution of reproductive output when lifespan was increased, although there was no reduction in lifetime reproduction. Quantifying reproduction is generally challenging in large cohorts of sexual taxa due to the possibility for mating effects on aging (e.g., Chapman and Partridge, ’96).
Here, we investigate resveratrol and food effects on lifespan and reproduction in Daphnia pulex. Daphnia has been a model species in ecology and evolutionary biology for over a century, and has recently emerged as a genomic model organism ideally suited for evaluating environmental effects on diverse traits (Eads et al., 2007; Colbourne et al., 2011; Dudycha et al., 2012). Effects of dietary restriction on lifespan have been known in Daphnia since the early 20th Century (Ingle et al., ’37), and natural genetic variation of aging has also been shown (Dudycha and Tessier, ’99; Dudycha, 2001; Dudycha, 2003). Unlike other invertebrate models of aging, Daphnia has adult tissue regeneration (Agar, ’30; Anderson, ’35) and therefore, like in humans, their pattern of aging is a balance between physiological deterioration and ongoing renewal.
Our study was initially motivated to examine the effects of resveratrol on lifespan and fecundity in the D. pulex TCO clone, of which the entire genome has been sequenced (Colbourne et al., 2011). Surprisingly, resveratrol had limited effects on TCO (see Results Section). This result led us to conduct another life table experiment to test whether survival and fecundity of TCO respond to a range of food levels in the same manner as other D. pulex clones respond to food level (Dudycha, 2003).
MATERIALS AND METHODS
Study Species
Daphnia are small crustaceans found in lakes and ponds throughout the world, and reproduce via cyclic parthenogenesis. In this life cycle, reproduction is normally via ameiotic cloning, with daughters genetically identical to their mothers. However, male development and sexual reproduction is triggered by periodic poor environmental conditions, and most populations therefore harbor substantial genetic variation. The advantage for demographic studies is that large, genetically uniform cohorts can be easily produced in the lab from a naturally occurring, evolutionarily successful genome. In addition, assessing female reproductive success is straightforward, since there is no need to manage and account for mating or sire effects.
Experimental Design
Our experiments were based on standard life table methods for Daphnia that have been detailed elsewhere (Dudycha and Tessier, ’99; Dudycha and Lynch, 2005). Life tables began with neonates younger than 12-hr old, collected from mothers that had been maintained at low density (1/100 mL) with satiating food for 2–3 weeks. At the start of a life table, each neonate was placed in a separate 150 mL beaker with 100 mL filtered (1 μm) lakewater collected from Lake Murray in Columbia, SC. Animals were transferred to beakers with fresh lakewater every 2 days. In order to prevent Daphnia from being trapped in the surface film, a small amount of cetyl alcohol was dusted on the surface (Desmarais, ’97). For all experiments, Daphnia were maintained in a controlled environment chamber with a 12:12 L:D cycle at 20°C, the laboratory environment to which TCO had been acclimated for several years (>50 generations) previously.
TCO individuals were grown at two different food levels (20,000 (“20K”) or 12,000 (“12K”) cells mL−1) of Ankistrodesmus falcatus fed daily. At the age of 9 days, the typical age at maturity for D. pulex (Dudycha, 2003), three resveratrol treatments (0 μM (control), 0.3 μM and 3 μM resveratrol) were applied to animals (“Resveratrol experiment” hereafter). Sixty TCO individuals were randomly assigned to each of two food levels and three resveratrol treatments, resulting in a total of 360 animals.
This experiment was designed based on results from a series of preliminary life table experiments. In those experiments, the control (0 μM) and low a dose of resveratrol (0.1 μM) were applied to animals fed with 12K or 20K cells mL−1 of A. falcatus. Three separate trials for each food level were conducted, and 30–40 individuals were assigned to each treatment in each trial. In this preliminary study, resveratrol did not affect lifespan except in one trial in which the median lifespan increased by 18% at 12K cells mL−1 of A. falcatus food level. Since the limited resveratrol effect may have been a consequence of resveratrol dose, we increased the concentrations of resveratrol to 0.3 and 3 μM in the experiment reported here. In addition, in the preliminary study, resveratrol treatment decreased fecundity in two trials at the 20K food level. Thus, animals at both 12K and 20K food levels were used in this experiment to evaluate resveratrol effects on fecundity as well as lifespan.
Resveratrol (Sigma–Aldrich, St. Louis, MO) was dissolved in ethanol, and 20 μL of ethanol or resveratrol–ethanol solution was added to 1 L filtered lakewater, making final concentrations of resveratrol of 0, 0.3, and 3 μM. Control and resveratrol treatments had the same concentration of ethanol (0.002% v/v). Since the activity of resveratrol can decrease in a solution during storage, fresh resveratrol–ethanol solution was prepared every 4 days.
Seven animals at the 12K (five in control, one in 0.3 μM resveratrol, and one in 3 μM resveratrol) and 10 animals at the 20K (three in control, one in 0.3 μM resveratrol, and six in 3 μM resveratrol) died as juveniles before resveratrol was applied (9th day). Since our purpose was to examine resveratrol effect on adults, these juvenile deaths were removed from the analysis.
An additional experiment was conducted in order to characterize survival and fecundity of the TCO clone in response to differential food levels (“Food experiment” hereafter). Five different concentrations of A. falcatus were added to lakewater media daily from the beginning of the experiment: 4,000 cells mL−1 (“4K”), 8,000 cells mL−1 (“8K”), 12,000 cells mL−1 (“12K”), 16,000 cells mL−1 (“16K”), and 20,000 cells mL−1 (“20K”). In this experiment, 250 animals were randomly assigned to each food treatment. The experiment was divided into three starting dates separated by 2 days, with 80–90 neonates for each treatment at each date.
Survivorship was recorded every 2 days until all animals died. Daphnia perform two types of reproduction: they usually produce immediately developing offspring via parthenogenesis, but can also produce diploid diapausing eggs encased in protective structures called ephippia. In order to evaluate fecundity, 30 individuals in each treatment of the resveratrol experiment and 45 individuals in each treatment for the food experiment were randomly selected at the beginning of life tables, and the number of immediately developing offspring and ephippia was counted every 2 days until they died.
Statistical Analyses
To assess treatment effects on longevity, log-rank tests were performed (SAS v. 9.2,), analyzing resveratrol and food experiments separately. Resveratrol treatments and food levels were included as strata in the models as appropriate, and survival functions were compared among strata. Significance of pairwise comparisons was adjusted based on Bonferroni method. For the food experiment, starting date did not affect survival curves (log-rank test, χ2 =1.13, df = 2, P = 0.57), so animals with different starting dates were pooled together for the analyses. In the resveratrol experiment, resveratrol effects seemed to become weaker at older age (see Results Section). As a post hoc analysis, three adult ontogenetic periods (early, middle, and late) were defined, and mortality hazard of each period and its SE was calculated: (i) early adult period from 9 days to 19 days, (ii) middle adult period from 20 days to 29 days, (iii) late adult period from 30 days to 39 days. By the end of the late adult period, fewer than 2% of individuals in each treatment survived in both the resveratrol experiment and the food experiment.
To compare fecundity among treatments, analysis of variance was conducted (SAS Proc GLM). The production of offspring fluctuates in Daphnia due to the ~2.4 day molt cycle (Dudycha and Tessier, ’99). In order to avoid statistical complexities due to such fluctuation, fecundity was evaluated in three adult periods that were used for survival analysis. Number of immediately developing offspring produced during a lifetime, and number of immediate offspring produced in each adult period were analyzed separately. For the resveratrol experiment, the resveratrol treatment, food level, and resveratrol by food interaction were independent variables. For the food experiment, the food treatment was the independent variable, and starting date was included as block. Fecundity was compared between each pair of treatments using a Bonferroni adjustment for multiple comparisons. Separate analyses of variance with the same model were conducted to compare number of ephippia across treatments in the resveratrol experiment and food experiment.
RESULTS
Resveratrol Experiment
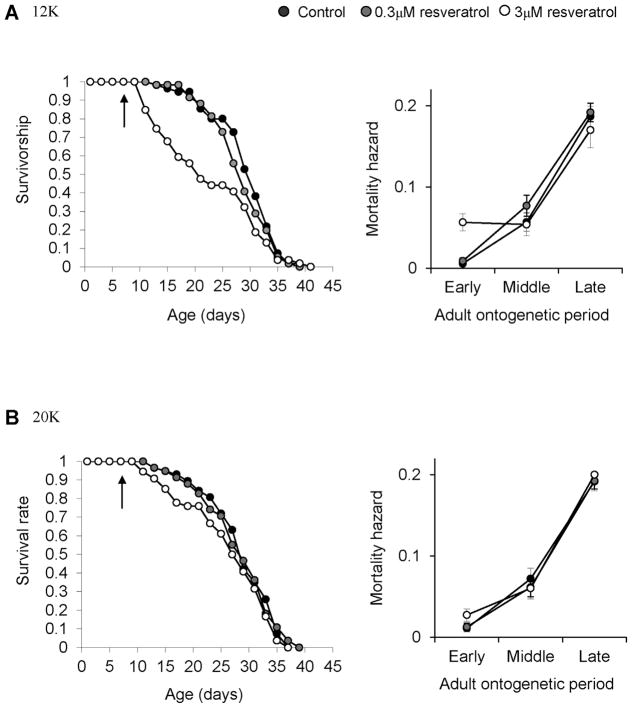
Resveratrol did not increase lifespan of the Daphnia TCO clone in this experiment (Fig. 1). Median lifespan of animals in 0.3 μM resveratrol treatment (12K, median lifespan = 29 days, 95% CI = (27, 31); 20K, median lifespan = 29 days, 95% CI = (27, 31)) was similar to controls at both 12K (median lifespan = 31 days, 95% CI = (29, 33), χ2 = 0.86, P = 1.00) and 20K (median lifespan = 29 days, 95% CI = (27, 31), χ2 = 0.01, P = 1.00) food levels. Notably, the highest dose of resveratrol (3 μM) decreased survival significantly at 12K (median lifespan = 21 days, 95% CI = (17, 29), χ2 = 9.03, P = 0.04) while no such effect was found at 20K (median lifespan = 28, 95% CI = (25, 33), χ2 =1.40 P = 1.00). This negative effect of resveratrol on survival was manifest only at the early adult period (Fig. 1).
Figure 1.
Survivorship of the Daphnia TCO strain in the resveratrol experiment. Survival curves during the experiment and mortality hazards with SEs at each adult period are given. Animals which died before the application of resveratrol were not included in the analyses. Survivorship and mortality hazard at 12K and 20K food levels are presented separately. Arrows in the survival curve indicate the age when resveratrol and control treatments started.
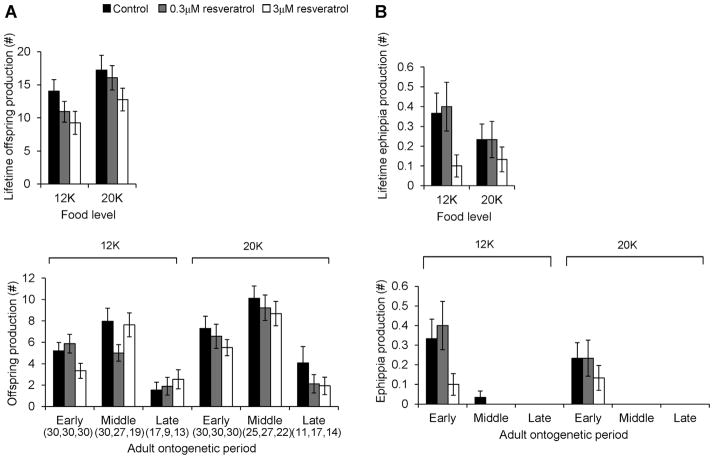
Both food level and resveratrol affected fecundity of the TCO clone (Fig. 2 and Table 1). Across resveratrol treatments, animals at 20K produced more offspring during their lifetime than those at 12K. In addition, animals in the higher resveratrol concentration tended to produce fewer offspring and ephippia during their lifetime compared to those in the control treatment. The resveratrol effect on fecundity was significant or marginally significant only at the early adult period (Table 1). No animals in the late adult period produced ephippia.
Figure 2.
Fecundity of the Daphnia TCO strain in the resveratrol experiment. Production of immediately developing offspring (A) and production of ephippia (B) are presented separately. Both lifetime fecundity and fecundity at each adult period are presented. Sample size is given within parenthesis. No ephippia were produced in the late adult period. Error bars show SE.
Table 1.
Results from analysis of variance to compare fecundity among treatments.
| Resveratrol experiment
|
Food experiment
|
|||
|---|---|---|---|---|
| Food (df = 1) | Resveratrol (df = 2) | Food x Resveratrol (df = 2) | Food (df = 5) | |
| Offspring production | ||||
| Lifetime | 7.05** | 3.23* | 0.16 | 25.07*** |
| Early adult period | 4.98** | 2.67+ | 0.42 | 27.69*** |
| Middle adult period | 7.15** | 1.61 | 1 | 17.50*** |
| Late adult period | 0.85 | 0.37 | 1.51 | 2.91* |
| Ephippia production | ||||
| Lifetime | 1.51 | 3.13* | 0.73 | 2.43* |
| Early adult period | 1.16 | 2.94+ | 0.66 | 2.11+ |
| Middle adult period | 0.67 | 0.72 | 0.72 | 0.67 |
| Late adult period | a | |||
For the resveratrol experiment, F ratios of food effect, resveratrol effect, and food by resveratrol interaction are given. For food experiment, F ratios of food effect are given. Significant effects are indicated by bold type:
P <0.1,
P <0.05,
P <0.01,
P <0.001.
No ephippia were produced in the late adult period.
Food Experiment
Food level significantly affected survival (χ2 = 69.37, P <0.001) (Fig. 3). In particular, animals at relatively high food levels (12K, 16K, 20K) survived better than those at 8K (8K vs. 12K, χ2 =10.23, P = 0.01; 8K vs. 16K, χ2 = 4.32, P = 0.38; 8K vs. 20K, χ2 = 8.97, P = 0.03) and at 4K (4K vs. 12K, χ2 = 45.12, P <0.001; 4K vs. 16K, χ2 = 30.81, P <0.001; 4K vs. 20K, χ2 = 42.11, P <0.001) food levels. A significant difference of mortality hazard between 20K and 4K was evident at both early and middle adult periods (Fig. 3).
Figure 3.
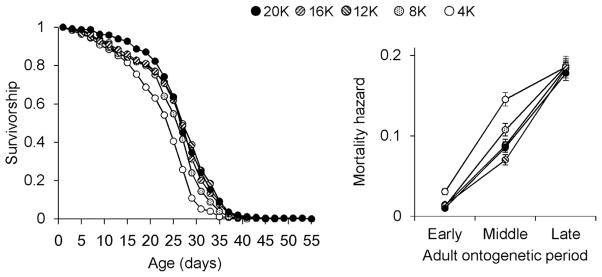
Survivorship of the Daphnia TCO strain in the food experiment. Survival curves during the experiment and mortality hazards with SEs at each adult period are presented.
TCO clones at higher food levels produced more offspring and ephippia during their lifetime. Food effects on offspring production were significant throughout all adult periods (Fig. 4, Table 1). Production of ephippia occurred mainly at the early adult life period.
Figure 4.
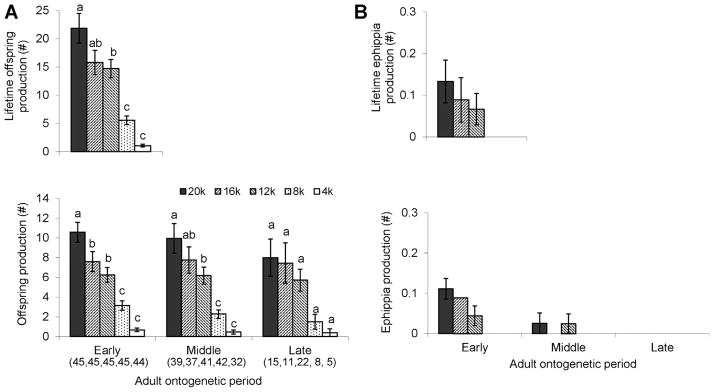
Fecundity of the Daphnia TCO strain in the food experiment. Production of immediately developing offspring (A) and production of ephippia (B) are presented separately. Both lifetime fecundity and fecundity at each adult period are presented. Sample sizes for the period-specific reproduction estimates are given in parentheses below the x-axis in (A). Note that the period averages do not sum to the lifetime averages due to deaths earlier than when the middle and late periods begin. Error bars represent SE. Different letters above bars indicate significant differences at P <0.05 based on pairwise comparisons using Bonferroni adjustment.
DISCUSSION
In this experiment, we examined responsiveness of Daphnia TCO to potentially related interventions that have been reported to extend lifespan: resveratrol and limited food level. Resveratrol decreased survival and fecundity at the early adult period soon after its application, while it did not influence survivorship and fecundity at the middle and late adult periods. Animals grown at relatively low food levels (4,000 cells mL−1 and 8,000 cells mL−1 A. falcatus) exhibited shorter median lifespan and lower lifetime fecundity compared to those at higher food levels (12,000 cells mL−1, 16,000 cells mL−1, and 20,000 cells mL−1 A. falcatus). This food level effect was significant throughout all adult ontogenetic periods.
Resveratrol did not extend lifespan of the Daphnia TCO clone. This is consistent with recent studies which showed no significant effect of resveratrol on the lifespan in yeast, nematode, fruit fly, and mouse systems (Bass et al., 2007; Kaeberlein et al., 2005; Miller et al., 2011). Notably, low food treatments did not extend lifespan in TCO either, suggesting that TCO might have distinctive physiological mechanisms responding to dietary restriction, which as a consequence might cause the absence of a resveratrol effect on lifespan.
When raised at the food level of 4,000 cells mL−1 or 8,000 cells mL−1 A. falcatus, TCO exhibited lower survival and fecundity than those raised at higher food levels, in sharp contrast to a previous study using genetically heterogeneous D. pulex cohorts (Dudycha, 2003). One possibility is that responses to dietary restriction are clone-specific, and TCO may be generally resistant to environmental influences on lifespan. TCO was chosen for genome sequencing because it is unusually homozygous, which likely originated through inbreeding. This high homozygosity may constrain the response of TCO to environmental variation if phenotypic plasticity is dependent on heterozygosity. It is also possible that the original study (Dudycha, 2003) contained a mixture of responsive and non-responsive genotypes. For instance, in Daphnia pulicaria, clonal variation in the response to dietary restriction exists: lifespan of some clones does not respond to food level, but the lifespan of other clones increased around 37% at 8,000 cells mL−1 A. falcatus compared to that at 20,000 cells mL−1 A. falcatus (Kim and Dudycha, unpublished data). Genetic polymorphism in animal responses to dietary restriction might occur in nature more commonly than previously appreciated, or the expression of responses may depend on genetic interactions with environmental factors that differ between the pond from which TCO was isolated and the lab environment.
The survival rate of TCO decreased rapidly after application of resveratrol at the 12K food level, suggesting a toxic, rather than protective, effect of resveratrol. A similar immediate decrease of survival after administration was also reported in a turquoise killifish species, Nothobranchius furzeri, in which relatively low doses of resveratrol (0.1 and 3 μM in food) was applied (Valenzano et al., 2006). No immediate effect of resveratrol was found in other model systems including mouse, fruit fly, and worm (Howitz et al., 2003; Wood et al., 2004; Baur et al., 2006). Physiological effects of resveratrol are dependent on the dose of application (Mukherjee et al., 2010), and extremely high doses of resveratrol have been implicated in shorter lifespan (Crowell et al., 2004; Pearson et al., 2008).
Relatively few studies have evaluated potential reproductive costs of resveratrol. Resveratrol suppressed lifetime fecundity of TCO at both 12K and 20K food levels. This effect was especially evident at the early adult period, when reproduction has the greatest impact on fitness. This result contrasts with previous studies in which resveratrol did not influence or even increased reproductive output in C. elegans and D. melanogaster (Wood et al., 2004), but partially matches the fecundity reduction observed in C. elegans (Gruber et al., 2007). While Gruber et al. (2007) found no effect on net lifetime fecundity, resveratrol treatment decreased offspring production at the early life stage and then increased offspring production at the late life stage. Although our data show a stronger deleterious effect of resveratrol on reproduction, they do not provide support for the notion that resveratrol may mediate a reproduction-survival tradeoff because we did not observe a corresponding lifespan extension.
In this study, resveratrol was applied at concentrations of 0.3 and 3 μM, which are relatively low when compared to studies applying 1–100 μM resveratrol in food. However, it is unlikely that the absence of lifespan extension was an effect of low dosage, because the application of 3 μM resveratrol showed a significant treatment effect on survival and fecundity at the early adult period. This indicates that the concentrations of resveratrol in this experiment are high enough to induce physiological changes in Daphnia. Furthermore, it is difficult to compare doses of resveratrol added to a food medium to those where it is added to a culture medium. Daphnia are filter feeders that continuously filter water throughout their life, and small molecules such as resveratrol can be absorbed across the gut lining (R. Patel, personal communication).
Even though our experiments did not show an effect of resveratrol on the lifespan of the TCO clone, it is not clear whether other Daphnia species or genotypes would have similar responses. For instance, D. pulicaria exhibit much longer lifespan than D. pulex at 20,000 cell mL−1 A. falcatus, and when both of them are grown at 3,000 cell mL−1 A. falcatus (i.e., a strong dietary restriction), D. pulicaria shows much stronger responses in lifespan compared to D. pulex (Dudycha, 2003). Thus, if resveratrol is a mimetic of dietary restriction, D. pulicaria might show significant responses to the resveratrol application. Gene sequences of sirtuins, a key protein responding to resveratrol, have diverged non-randomly between D. pulex and D. pulicaria lineages, suggesting possibly distinctive molecular characteristics between D. pulex and D. pulicaria sirtuins (J. Dudycha and P. Li, unpublished data).
Overall, our results showed moderate negative effects of resveratrol on reproduction, but no net effects on the lifespan of the Daphnia TCO clone. Similarly, dietary restriction did not extend the lifespan of TCO but did limit reproductive output. If resveratrol activates genes involved in the response to dietary restriction, our results suggest that effects of resveratrol will also depend on genetic polymorphisms that lead to differential responses to dietary restriction. A handful of published studies have failed to show an extension of lifespan with resveratrol (see Bass et al., 2007; Miller et al., 2011), and a number of studies have suggested that the lifespan extension of dietary restriction is dependent on the genotypes studied (e.g., Liao et al., 2010). Although the principle of dietary restriction extending lifespan may be broadly conserved across taxa, it appears that this is subject to relatively frequent modification to a life history that is more canalized against environmental variation. This indicates that the connection between diet and aging is not immutable and is consistent with current thinking that multiple molecular mechanisms are involved in the dietary restriction response.
Acknowledgments
We appreciate the comments of Chris Brandon and four anonymous reviewers on an earlier version of this manuscript. Our work was funded by NIH grant R01AG037969.
LITERATURE CITED
- Agar WE. A statistical study of regeneration in two species of Crustacea. J Exp Biol. 1930;7:349–369. [Google Scholar]
- Anderson BG. Antennal regeneration in Daphnia magna. Ohio J Sci. 1935;35:105–111. [Google Scholar]
- Barger JL, Kayo T, Vann JM, et al. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS ONE. 2008;3:e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass TM, Weinkove D, Houthoofd K, Gems D, Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech Ageing Dev. 2007;128:546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop NA, Guarente L. Genetic links between diet and lifespan: shared mechanisms from yeast to humans. Nat Rev Genet. 2007;8:835–844. doi: 10.1038/nrg2188. [DOI] [PubMed] [Google Scholar]
- Burnett C, Valentini S, Cabreiro F, et al. Absence of effects of sir2 overexpression on lifespan in C. elegans and Drosophila. Nature. 2011;477:482–485. doi: 10.1038/nature10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chachay VS, Kirkpatrick CMJ, Hickman IJ, et al. Resveratrol—pills to replace a healthy diet? Br J Clin Pharmacol. 2011;72:27–38. doi: 10.1111/j.1365-2125.2011.03966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekara KT, Shakarad MN. Aloe vera or resveratrol supplementation in larval diet delays adult aging in the fruit fly, Drosophila melanogaster. J Gerontol A Biol Sci Med Sci. 2012;66A:965–971. doi: 10.1093/gerona/glr103. [DOI] [PubMed] [Google Scholar]
- Chapman T, Partridge L. Female fitness in Drosophila melanogaster: an interaction between the effect of nutrition and of encounter rate with males. Proc R Soc Lond B. 1996;263:755–759. doi: 10.1098/rspb.1996.0113. [DOI] [PubMed] [Google Scholar]
- Colbourne JK, Pfrender ME, Gilbert D, et al. The ecoresponsive genome of Daphnia pulex. Science. 2011;331:555–561. doi: 10.1126/science.1197761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell JA, Korytko PJ, Morrissey RL, Booth TD, Levine BS. Resveratrol-associated renal toxicity. Toxicol Sci. 2004;82:614–619. doi: 10.1093/toxsci/kfh263. [DOI] [PubMed] [Google Scholar]
- Desmarais KH. Keeping Daphnia out of the surface film with cetyl alcohol. J Plankt Res. 1997;19:149–154. [Google Scholar]
- Dudycha JL. The senescence of Daphnia from risky and safe habitats. Ecol Lett. 2001;4:102–105. [Google Scholar]
- Dudycha JL. A multi-environment comparison of senescence between sister species of Daphnia. Oecologia. 2003;135:555–563. doi: 10.1007/s00442-003-1230-7. [DOI] [PubMed] [Google Scholar]
- Dudycha JL, Lynch M. Conserved ontogeny and allometric scaling of resource acquisition and allocation in the Daphniidae. Evolution. 2005;59:565–576. [PubMed] [Google Scholar]
- Dudycha JL, Tessier AJ. Natural genetic variation of life span, reproduction, and juvenile growth in Daphnia. Evolution. 1999;53:1744–1756. doi: 10.1111/j.1558-5646.1999.tb04559.x. [DOI] [PubMed] [Google Scholar]
- Dudycha JL, Brandon CS, Deitz KC. Population genomics of resource exploitation: insights from gene expression profiles of two Daphnia ecotypes fed alternate resources. Ecol Evol. 2012;2:329–340. doi: 10.1002/ece3.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eads BD, Andrews J, Colbourne JK. Ecological genomics in Daphnia: stress responses and environmental sex determination. Heredity. 2007;100:184–190. doi: 10.1038/sj.hdy.6800999. [DOI] [PubMed] [Google Scholar]
- Finch CE, Tanzi RE. Genetics of aging. Science. 1997;278:407–411. doi: 10.1126/science.278.5337.407. [DOI] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span—from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber JAN, Tang SY, Halliwell B. Evidence for a trade-off between survival and fitness caused by resveratrol treatment of Caenorhabditis elegans. Ann NY Acad Sci. 2007;1100:530–542. doi: 10.1196/annals.1395.059. [DOI] [PubMed] [Google Scholar]
- Harper JM, Leathers CW, Austad SN. Does caloric restriction extend life in wild mice? Aging Cell. 2006;5:441–449. doi: 10.1111/j.1474-9726.2006.00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Ingle L, Wood TR, Banta AM. A study of longevity, growth, reproduction and heart rate in Daphnia longispina as influenced by limitations in quantity of food. J Exp Zool. 1937;76:325–352. [Google Scholar]
- Kaeberlein M, McDonagh T, Heltweg B, et al. Substrate-specific activation of sirtuins by resveratrol. J Biol Chem. 2005;280:17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- Knutson MD, Leeuwenburgh C. Resveratrol and novel potent activators of sirt1: effects on aging and age-related diseases. Nutr Rev. 2008;66:591–596. doi: 10.1111/j.1753-4887.2008.00109.x. [DOI] [PubMed] [Google Scholar]
- Lee GD, Wilson MA, Zhu M, et al. Dietary deprivation extends lifespan in Caenorhabditis elegans. Aging Cell. 2006;5:515–524. doi: 10.1111/j.1474-9726.2006.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C-Y, Rikke BA, Johnson TE, Diaz V, Nelson JF. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell. 2010;9:92–95. doi: 10.1111/j.1474-9726.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W, Dillin A. Aging and survival: the genetics of life span extension by dietary restriction. Annu Rev Biochem. 2008;77:727–754. doi: 10.1146/annurev.biochem.77.061206.171059. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66A:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockett R, Cooper TM, Orr W, Sohal R. Effects of caloric restriction are species-specific. Biogerontology. 2006;7:157–160. doi: 10.1007/s10522-006-9004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Dudley JI, Das DK. Dose-dependency of resveratrol in providing health benefits. Dose–Response. 2010;8:478–500. doi: 10.2203/dose-response.09-015.Mukherjee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan SD, Yen K, Tissenbaum HA. Converging pathways in lifespan regulation. Curr Biol. 2009;19:R657–R666. doi: 10.1016/j.cub.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacholec M, Bleasdale JE, Chrunyk B, et al. Srt1720, srt2183, srt1460, and resveratrol are not direct activators of sirt1. J Biol Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L, Pletcher SD, Mair W. Dietary restriction, mortality trajectories, risk and damage. Mech Ageing Dev. 2005;126:35–41. doi: 10.1016/j.mad.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzano DR, Terzibasi E, Genade T, et al. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]