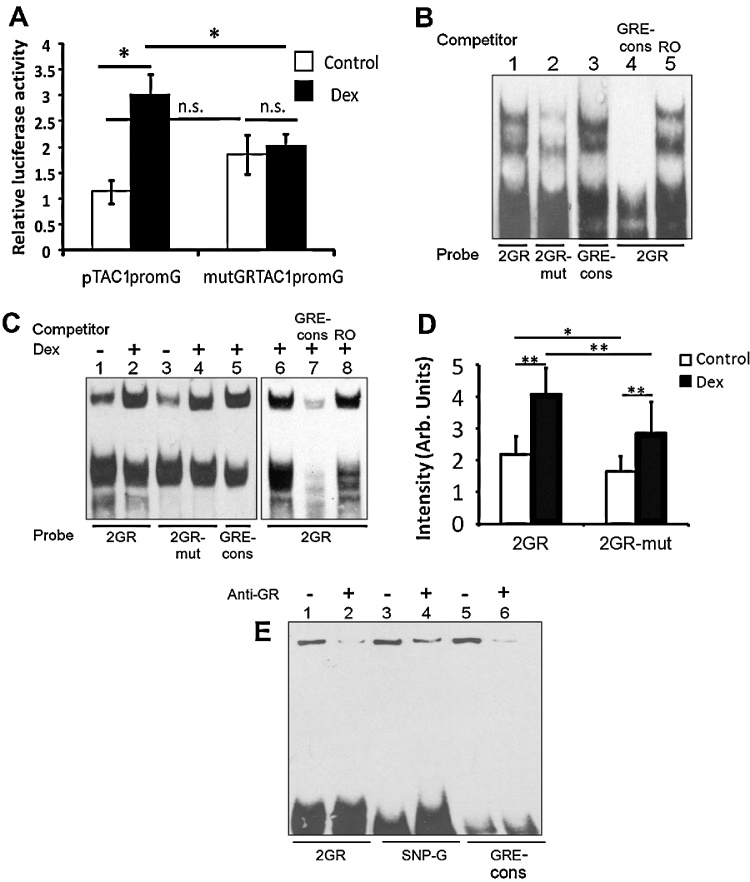
Figure 3.
(A) Relative luciferase gene expression driven by either the pTAC1promG-luc or mut2GRTAC1prom-Luc plasmids when transfected into primary amygdala neurones treated with vehicle or Dex (n = 9). (B) EMSA analysis of purified recombinant human GRα protein incubated with labelled oligonucleotide probes representing wild type 2GR (2GR); mutated 2GR (2GR-mut) or consensus MMTV-LTR glucocorticoid response element (GRE-cons) and (above) 100 fold molar excess of unlabelled competing GRE-cons oligonucleotides or random oligonucleotide (RO). (C) Lanes 1–6; EMSA analysis of labelled 2GR, 2GR-mut or GRE-cons oligonucleotides incubated with nuclear extracts derived from SH-SY5Y cells treated with vehicle or Dex. Lanes 7 and 8; competitive gel shift assays carried out with competing GRE-cons or RO. (D) Densometric analysis of high molecular weight DNA:protein complexes in lanes 1–4, significance: *p < 0.05; **p < 0.01. (E) Lanes 1–6, nuclear extract from SH-SY5Y cells previously incubated in Dex and incubated with labelled probe representing the 2GR, SNP-G or the GR binding consensus sequence (GRE-cons) after pre-incubation with rabbit pre-immune serum (−) or antibody (+). 2GR (Lanes 1 and 2), SNPGR-G (SNP-G; Lanes 3 and 4) and GRE-cons (Lane 5 and 6) were incubated with SH-SY5Y nuclear extracts in the presence or absence of Anti-GR antibody that reduced GR binding to each of the probes. These EMSAs were exposed to electric fields for an additional 30 min to facilitate resolution of high molecular weight DNA:protein complexes.