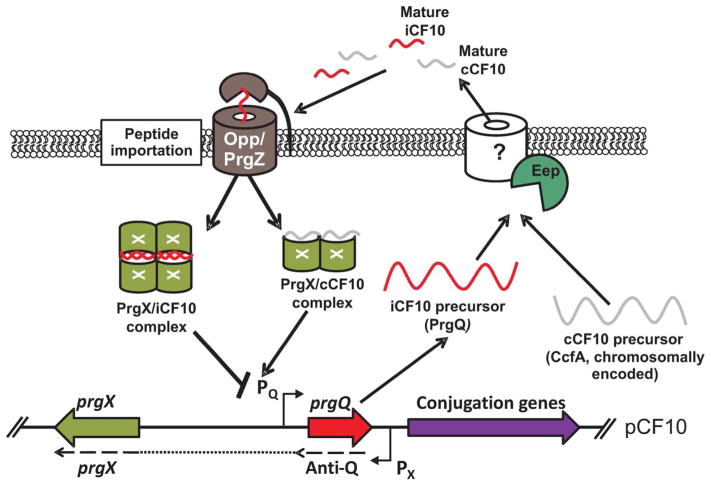
Figure 3. Peptide regulation of pCF10 conjugation in E. faecalis.
During non-inducing conditions, PrgQ is produced constitutively from the PQ promoter. PrgQ, the precursor for the iCF10 peptide, is processed by Eep immediately before or during secretion. Mature iCF10 pheromone in the extracellular milieu is sensed by surrounding cells and taken up via PrgZ and the Opp system where the peptide interacts with the PrgX regulator. PrgX-iCF10 complexes are thought to form a tetrameric structure allowing binding of PrgX to DNA adjacent to PQ, effectively shutting off transcription by blocking RNA polymerase access. Concurrently, cells are constitutively producing CcfA from the chromosome. CcfA, the cCF10 peptide precursor, is processed, secreted, and reimported similarly to iCF10. Once exported, mature cCF10 pheromone has been re-internalized, it competes with iCF10 to bind to PrgX. When cCF10 is in abundance, the system favors PrgX-cCF10 complexes destabilizing the DNA binding tetramer and allowing for increased RNA polymerase access to PQ and transcription of the downstream conjugation genes.