Abstract
IL-6 is a pleiotropic cytokine that exerts either proinflammatory or anti-inflammatory effects, and is implicated in diverse settings including obesity, exercise, arthritis, and colitis. A new study shows that modulation of macrophage activation by IL-6 maintains glucose homeostasis in diet-induced obesity while limiting inflammation in endotoxemia (Mauer et al., 2014).
IL-6 is generally considered a pro-inflammatory cytokine as it promotes neutrophilia and TH17 T cell differentiation while blocking regulatory T cell (T reg) differentiation. IL-6 critically contributes to pathophysiology of several inflammatory diseases, including rheumatoid arthritis and experimental autoimmune encephalomyelitis. However, an immunoregulatory role for IL-6 has also been suggested in specific contexts. For instance, genetic deficiency of IL-6 increases inflammatory responses to local and systemic endotoxin administration. Muscle-derived IL-6 may also mediate some of the anti-inflammatory and insulin-sensitizing effects of physical exercise. Thus, available evidence is consistent with pleiotropic functions for the cytokine (Scheller et al., 2011). Importantly, how IL-6 regulates macrophage biology remains not well understood. This is in striking contrast to IL-10, a cytokine that signals similarly to IL-6 and yet serves as a paradigm for immunoregulation of macrophage activation. In a new study, Mauer et al. make the unexpected and important finding that, while IL-6 potentiates alternative (M2) macrophage activation by IL-4, resulting in improved metabolic responses to high-fat diet challenge, it attenuates classical (M1) macrophage activation to LPS, thus conferring protection to endotoxemia (Mauer et al., 2014).
Mauer et al. first investigated the role of IL-6 in controlling macrophage activation in the context of metabolic homeostasis, where the modality of macrophage activation is known to be critical. M2 activation of adipose tissue macrophages, mediated by IL-4 and/or IL-13 production from eosinophils and/or ILC2 cells, favors insulin sensitivity. On the other hand, M1 activation, triggered by saturated fatty acids and/or adipocyte derived inflammatory cytokines, contributes to obesity-associated chronic inflammation (or metaflammation), insulin resistance, and systemic metabolic deterioration (Chawla et al., 2011; Gregor and Hotamisligil, 2011). The authors found that despite comparable food intake and weight gain, mice with myeloid-specific deletion of IL-6Ra (IL6raΔmyel) were more glucose intolerant and insulin resistant on a high fat diet. This may be a result of increased metaflammation in white adipose tissue, brown adipose tissue and liver, as indicated by increased expression of markers of M1 macrophages, while expression of M2 markers was reduced (Mauer et al., 2014).
To elucidate the basis for such modulation of macrophage activation, the authors used microarray analysis to identify IL-6-regulated responses (Mauer et al., 2014). Interestingly, IL-4ra, which encodes the IL-4 receptor binding both IL-4 and IL-13, emerged as one of the most strongly inducible genes in IL-6-stimulated bone marrow derived macrophages (BMDMs), and was identified as a direct target of Stat3, the major transcription factor activated by IL-6 signaling. Accordingly, IL-6 increased IL-4-mediated induction of multiple M2 genes in vitro and in vivo. In addition, IL-4ra expression in WAT was reduced in obese IL6raΔmyel mice, which dovetails nicely with the relative inability of exogenous IL-4 to improve glucose tolerance in these mice. Conversely, Mauer et al. showed that IL-6 exerts opposing effects on M1 activation, since pretreatment of BMDMs with IL-6 attenuated LPS-mediated induction of multiple genes, including TNFα and Nos2 (Mauer et al., 2014). Indeed, IL6raΔmyel mice were more susceptible to LPS-induced endotoxemia, as indicated by increased plasma levels of proinflammatory cytokines, and exacerbated weight loss and reduced food intake. Collectively, these findings establish a role for IL-6 in promoting M2 activation while attenuating M1 activation (Figure).
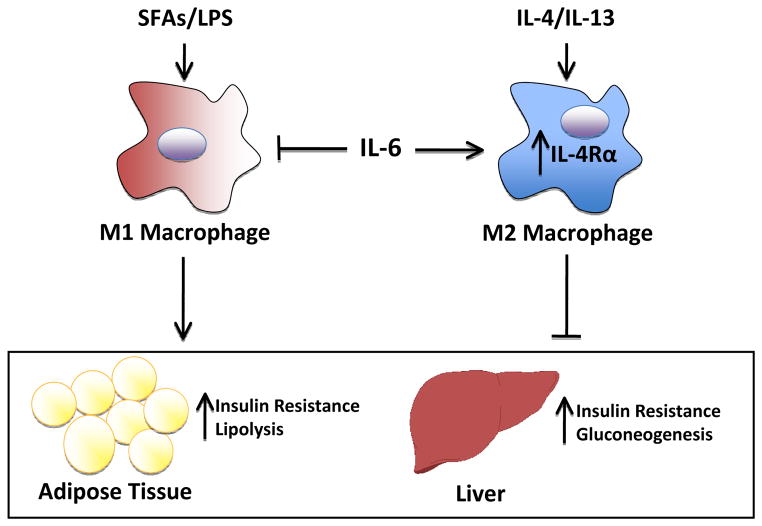
Figure. IL-6 limits obesity-induced insulin resistance by promoting macrophage M2 polarization.
In the obese state, increased production of IL-6 promotes M2 activation of adipose tissue macrophages by upregulating the expression of IL-4Ra. In parallel, IL-6 may inhibit the effects of saturated fatty acids (SFAs) and LPS (lipopolysaccharide) in triggering M1 activation. Thus IL-6 regulates activation of adipose tissue macrophages to attenuate inflammatory responses, improve insulin sensitivity, and resist metabolic dysregulation.
The study by Mauer et al. suggests that IL-6 may modulate macrophage activation during diet-induced obesity to attenuate inflammatory responses and resist metabolic dysregulation. In the obese state, increased IL-6 production (Pedersen and Febbraio, 2007) may be an adaptive response to metabolic stress. Because IL-6 is produced by many cell types and tissues and secreted into the circulation, the potential sources are manifold and could include adipocytes and the liver. Such IL-6 production potentiates M2 activation of adipose tissue macrophages by IL-4 and presumably IL-13, which also signals through IL-4Rα. In parallel, IL-6 may dampen M1 activation by saturated fatty acids and LPS, and perhaps other inflammatory cytokines, all of which are elevated in obesity. Thus IL-6 may be produced as a negative feedback mechanism during chronic nutrient excess to tune macrophage activation (Figure). The complex and nuanced functions of IL-6 may explain the conflicting literature on its role in metabolic regulation, with some studies supporting beneficial effects while others indicating detrimental activities (Pedersen and Febbraio, 2007). For example, IL-6-deficient mice develop mature onset obesity in one study but not another, while acute infusion of IL-6 can impair insulin sensitivity or enhance glucose tolerance. Of note, the emerging view of IL-6 as both an immunoregulatory and pro-inflammatory cytokine is consistent with its dual roles in catabolic (e.g. increasing lipolysis) and anabolic (e.g. increasing insulin sensitivity) metabolism. While the complexities of IL-6 function will take more time to sort out, one intriguing possibility is that its source, target and circulating levels can determine its pro-inflammatory versus immunoregulatory effects. In this regard, it has been proposed that IL-6 signaling via the classical pathway (through IL-6Rα and gp130) and in trans (by binding of a IL-6 and soluble IL-6Rα complex to cell surface gp130) may lead to different outcomes (Scheller et al., 2011).
The findings of Mauer et al. raise additional interesting points for discussion. While IL-4Rα upregulation would undoubtedly sensitize macrophages to IL-4, IL-6 is likely to promote M2 activation via additional mechanisms, for example via AMPK and/or PI3K/Akt activation which are implicated in macrophage polarization to a M2 or anti-inflammatory phenotype. Such mechanisms could allow for modulation of specific subsets of M2 genes, as opposed to IL-4ra induction, which may increase induction of all M2 genes. Regardless of the underlying mechanism, knowing whether IL-6 synergizes with IL-4 in the induction of a subset or the entirety of the M2 program may offer additional insights into the rationale of M2 activation by IL-6. How IL-6 negatively regulates M1 activation would also be important to explore. Finally, does IL-6 regulate M2 activation in other physiological and pathophysiological contexts? Exercise, cold stress, and fasting have all been shown to increase circulating levels of IL-6, and M2 activation in these contexts may promote muscle regeneration, adaptive thermogenesis, and lipid scavenging, respectively (Kosteli et al., 2010; Nguyen et al., 2011; Pillon et al., 2013; Wernstedt et al., 2006; Wueest et al., 2014). Related to this, IL-6 can stimulate whole body and intramyocellular fatty acid oxidation as well as lipolysis (Pedersen and Febbraio, 2007). Since beta-oxidation supports M2 activation (as opposed to glycolytic metabolism in the case of M1 macrophages)(Chawla et al., 2011), such effects of IL-6 would enable coordination of M2 activation with the systemic metabolic profile.
Despite its discovery nearly 30 years ago, how IL-6 controls macrophage biology has remained poorly understood. The study by Mauer et al. highlights the role of IL-6 as an important regulator of macrophage activation, and is sure to spark new interest in this fascinating cytokine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Selected Reading
- Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nature reviews. Immunology. 2011;11:738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annual review of immunology. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, Ferrante AW., Jr Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. The Journal of clinical investigation. 2010;120:3466–3479. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD, Theurich S, Hausen AC, Schmitz J, Bronneke HS, et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nature immunology. 2014;15:423–430. doi: 10.1038/ni.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. Point: Interleukin-6 does have a beneficial role in insulin sensitivity and glucose homeostasis. Journal of applied physiology. 2007;102:814–816. doi: 10.1152/japplphysiol.01208.2006. [DOI] [PubMed] [Google Scholar]
- Pillon NJ, Bilan PJ, Fink LN, Klip A. Cross-talk between skeletal muscle and immune cells: muscle-derived mediators and metabolic implications. American journal of physiology Endocrinology and metabolism. 2013;304:E453–465. doi: 10.1152/ajpendo.00553.2012. [DOI] [PubMed] [Google Scholar]
- Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochimica et biophysica acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- Wernstedt I, Edgley A, Berndtsson A, Faldt J, Bergstrom G, Wallenius V, Jansson JO. Reduced stress- and cold-induced increase in energy expenditure in interleukin-6-deficient mice. American journal of physiology Regulatory, integrative and comparative physiology. 2006;291:R551–557. doi: 10.1152/ajpregu.00514.2005. [DOI] [PubMed] [Google Scholar]
- Wueest S, Item F, Boyle CN, Jirkof P, Cesarovic N, Ellingsgaard H, Boni-Schnetzler M, Timper K, Arras M, Donath MY, et al. Interleukin-6 contributes to early fasting-induced free fatty acid mobilization in mice. American journal of physiology. Regulatory, integrative and comparative physiology. 2014 doi: 10.1152/ajpregu.00533.2013. [DOI] [PubMed] [Google Scholar]