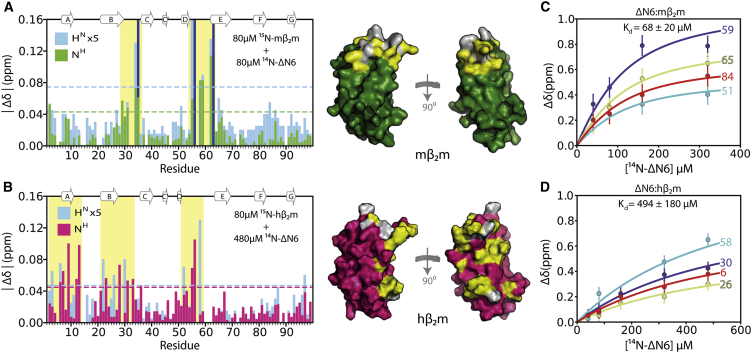
Figure 3.
Chemical Shift Changes and Binding Affinities for Different Complexes
(A) Chemical shift differences (1H, cyan; 15N, green) when 15N-labeled mβ2m and 14N-labeled ΔΝ6 are mixed in a 1:1 ratio (80 μΜ each; ∼41% mβ2m-bound). All residues experiencing significant chemical shift differences (yellow boxes) locate to the top half of the molecule (BC and DE loops; highlighted in yellow on the surface of the molecule; right-hand side). Residues that show large chemical shift differences in the presence of 40 μΜ ΔΝ6 but are broadened beyond detection at these protein concentrations are marked with dark blue bars.
(B) As in (A) but for 15N-labeled hβ2m and 14N-labeled ΔΝ6 mixed in a 1:6 ratio (80 μΜ hβ2m; 480 μM ΔΝ6; ∼47% hβ2m-bound). Residues with missing assignments are colored gray on the structure of mβ2m/hβ2m and have missing bars in (A) and (B). Dotted lines in (A) and (B) represent two standard deviations of the mean over the entire data set for each atom type.
(C) Plots of the chemical shifts of different residues (51, 59, 65, 84) in 15N-labeled mβ2m upon titration with 14N-labeled ΔN6. Solid lines represent global fits to a binding hyperbola. Error bars were calculated using resonances known not to be involved in the binding interface. For these residues the chemical shift was measured in each spectrum, and the error bars represent the standard deviation of the mean of their peak positions (see Experimental Procedures and Supplemental Experimental Procedures).
(D) As in (C) but for 15N-labeled hβ2m upon titration with 14N-labeled ΔN6. Curves for residues 6, 26, 30, and 58 are shown.