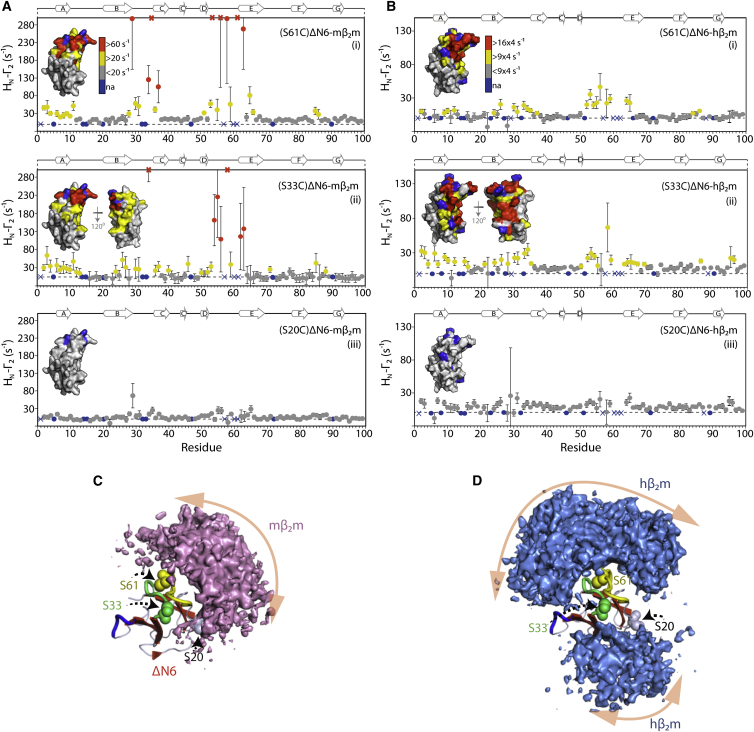
Figure 4.
Interaction Interfaces in Different Protein Complexes
(A) Per-residue Γ2 rates of mβ2m (60 μΜ) when MTSL is attached to S61 (i), S33 (ii), or S20 (iii) on ΔN6 (60 μΜ) colored according to their amplitude (blue, not assigned; gray, insignificant; yellow, >20 s−1; red, >60 s−1; pH 6.2, 25°C). The structure of mβ2m as a surface representation colored by the amplitude of the Γ2 rates is shown (insets). Red crosses indicate residues for which the Γ2 rate is either too large to appear on this scale or resonances broadened beyond detection when the spin label is oxidized and hence the Γ2 rate cannot be measured. Blue dots represent proline or overlapping resonances, and blue crosses denote residues for which the assignments are missing. Error bars were calculated from the noise level in the experiment.
(B) As in (A) but for the interaction between 14N- and MTSL-labeled ΔΝ6 (60 μΜ) and 15N-labeled hβ2m (60 μΜ). The structure of hβ2m is colored according to the amplitude of the Γ2 rates after extrapolation to the same % bound as in (A) (blue, not assigned; gray, insignificant; yellow, >9 × 4 s−1; red, >16 × 4 s−1). Note that the scale is expanded in (B).
(C) The distribution of the mβ2m molecules in the ΔN6-mβ2m complex, with the mβ2m ensemble shown as a pink surface around ΔΝ6 (cartoon). The 50 top-scoring ensembles (N = 2, 2 × 50 structures) were included in the calculation.
(D) As in (C) but for the ΔΝ6-hβ2m association. The pose of ΔΝ6 is identical to (C) and the ensemble of hβ2m subunits is colored in blue. The BC, DE, and FG loops of ΔΝ6 are highlighted in green, yellow, and blue, respectively, and the positions of the spin label (S20, S33, and S61) are shown as spheres.