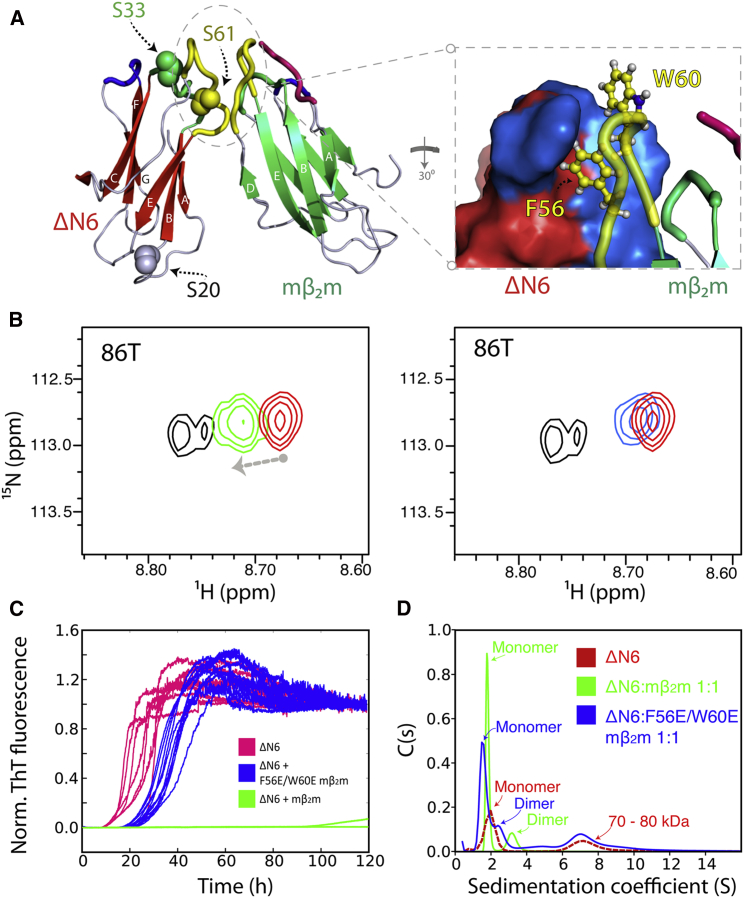
Figure 5.
F56 and W60 in mβ2m Form Interactions Required for Amyloid Inhibition
(A) The lowest-energy calculated structure of the ΔN6 (red)-mβ2m (green) complex highlighting F56 and W60 in the interface. Interface residues are colored blue on ΔΝ6 (right).
(B) Representative sample resonances in the 1H-15N HSQC spectrum of 15N-labeled ΔΝ6 (80 μM; red) that show chemical shift changes upon the addition of 14N-labeled mβ2m (green) but not its F56E/W60E variant (160 μM; blue). Addition of mβ2m shifts the resonances of ΔΝ6 toward their positions at pH 8.2 (black), where ΔN6 is not amyloidogenic (Eichner et al., 2011) (additional examples are shown in Figure S5A).
(C) Fibrillation kinetics of ΔΝ6 alone (20 μΜ; pink) at pH 6.2 and in the presence of a 2-fold molar excess of mβ2m (green) or F56E/W60E mβ2m (blue).
(D) Sedimentation velocity AUC traces of ΔΝ6 alone (60 μM; red), ΔΝ6 (60 μM) mixed with an equimolar concentration of mβ2m (green), or F56E/W60E mβ2m (blue).