Figure 1.
Characterization of a Bag6-Dependent Ligase for Mislocalized Proteins
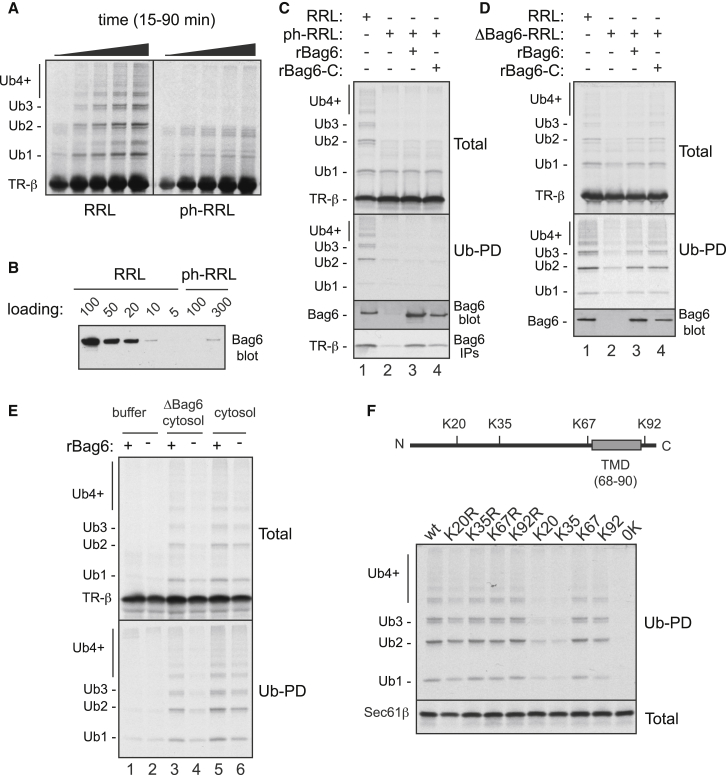
(A) 35S-methionine-labeled TR-β was in vitro translated for various times using rabbit reticulocyte lysate (RRL, left) or phenyl-depleted RRL (ph-RRL, right) and analyzed by SDS-PAGE and autoradiography. Unmodified TR-β and its ubiquitinated species are indicated.
(B) Levels of Bag6 in different relative amounts of RRL and ph-RRL were determined by immunoblot.
(C) Radiolabeled TR-β was produced in RRL, ph-RRL, or ph-RRL supplemented with recombinant Bag6 (rBag6) or Bag6 complex (rBag6-C). All reactions contained His-tagged ubiquitin. The translation products were analyzed directly (Total) or after His-ubiquitin pull-downs (Ub-PD). The level of Bag6 in each reaction was measured by immunoblotting (Bag6 blot). Bag6 interaction with TR-β was assessed by visualizing the amount of radiolabeled TR-β in anti-Bag6 immunoprecipitations (Bag6 IPs).
(D) TR-β was translated in control (RRL) or Bag6-depleted (ΔBag6-RRL) lysates without or with readdition of rBag6 or rBag6-C as indicated. Reactions were analyzed directly by SDS-PAGE and autoradiography (Total) or after ubiquitin pull-downs (Ub-PD). Bag6 in each reaction was analyzed by immunoblot.
(E) Radiolabeled TR-β translated for 30 min in ph-RRL in the presence (+) or absence (−) of rBag6 was subsequently incubated with buffer, total RRL (cytosol), or Bag6-depleted RRL (ΔBag6 cytosol). TR-β ubiquitination was followed by SDS-PAGE and autoradiography directly (Total) or after ubiquitin pull-downs (Ub-PD).
(F) Diagram depicting location of lysines and transmembrane domain (TMD) in Sec61β. Wild-type Sec61β, lysine-to-arginine mutants (KxR, where x denotes residue number) or constructs containing a single lysine (Kx, where x denotes residue number of the single lysine) were in vitro translated and analyzed directly (Total) or after ubiquitin pull-downs (Ub-PD).
See also Figure S1.