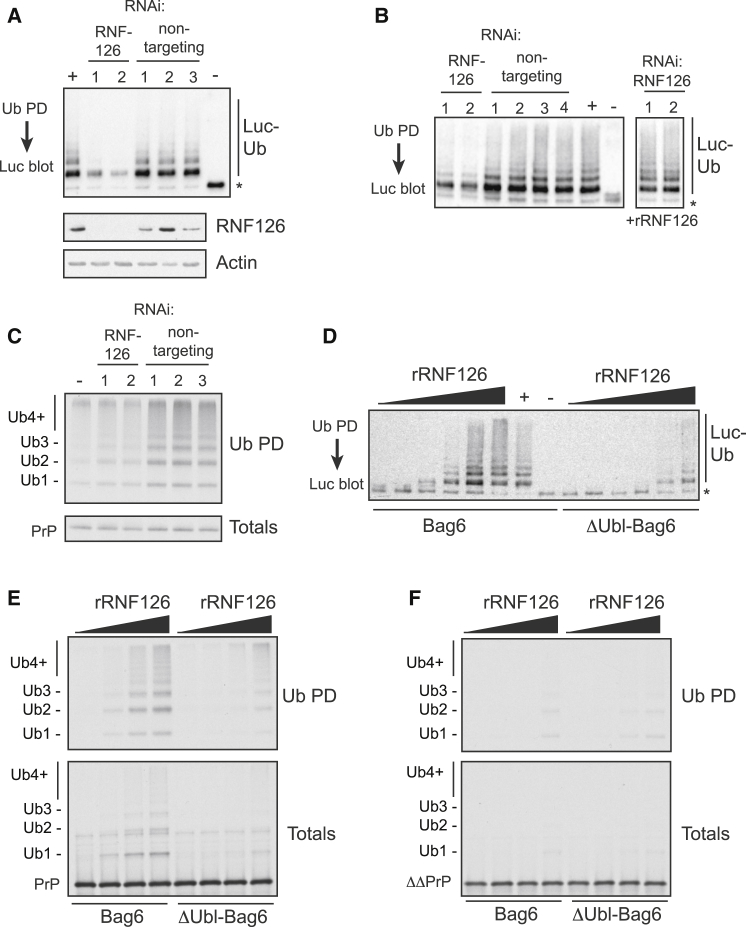
Figure 4.
RNF126 Is Necessary and Sufficient for Bag6-Mediated Client Ubiquitination
(A) Lysates prepared from HEK293T cells treated with the indicated siRNAs were used in ubiquitination assays of Bag6-Luc (top panel). The same lysates were immunoblotted for RNF126 (middle) or Actin (bottom). Untransfected cells were used as positive control for ubiquitination (+). The negative reaction (−) contains all components except lysate.
(B) Analysis similar to in (A), with recombinant RNF126 (rRNF126, 0.1 μM final concentration) added prior to the assay to lysates 1 and 2 where indicated.
(C) PrP was translated in phenyl-depleted lysate supplemented with recombinant Bag6, and this sample was used as the substrate in ubiquitination assays of HEK293T lysates as in (A). The reaction products were analyzed directly (Total) or after ubiquitin pull-downs (Ub-PD).
(D) Various amounts of rRNF126 (final concentrations of 0.3, 1, 3.1, 9.8, 31, and 98 nM) were added to reactions containing E1, E2, ATP, Flag-ubiquitin, and either Bag6-Luc complex (Bag6) or ΔUbl-Bag6-Luc complex (ΔUbl-Bag6). Ubiquitinated products were immunoprecipitated via Flag resin and immunobloted for luciferase. As a positive control, RRL was used as the source of the ligase (+).
(E) PrP was translated in phenyl-depleted lysate supplemented with recombinant Bag6 or ΔUbl-Bag6 in the presence of increasing amounts of rRNF126 (final concentrations of 0, 12, 39, and 118 nM). The translation products were analyzed directly (Total) or after ubiquitin pull-downs (Ub-PD).
(F) Assay as in (E), but using ΔΔPrP as the substrate.
See also Figure S4.