Abstract
The androgen receptor (AR) is critical for the normal development of prostate and for its differentiated functions. The consistent expression of AR in prostate cancer (PCa), and its continued activity in PCa that relapse after androgen deprivation therapy (castration-resistant prostate cancer (CRPC)), indicate that at least a subset of these genes are also critical for PCa development and progression. This review addressed AR regulated genes that may be critical for PCa, and how AR may acquire new functions during PCa development and progression.
AR is a steroid receptor and member of the nuclear receptor family of ligand-activated transcription factors. It has a large N-terminal domain that can strongly stimulate transcription, a C-terminal ligand-binding domain (LBD) that has a weaker transactivation function, a central DNA-binding domain and a short hinge region between the DNA binding domain and LBD that mediates functions including its nuclear translocation and degradation. In the absence of androgen (testosterone or dihydrotestosterone), the AR associates with an HSP90 chaperone complex in the cytoplasm. In response to androgen binding to the LBD, the AR undergoes a conformational change that repositions helix 12 to generate a binding site for LXXLL-motifs found in many coactivator proteins. Interestingly, in the AR this coactivator binding site initially binds to an LXXLL-like motif in the AR N-terminal domain, which may be important for nuclear translocation or the initial steps in chromatin binding. The liganded AR then forms a homodimer in the nucleus and binds to regulatory regions of multiple genes encoding proteins (such as prostate-specific antigen) that are critical for prostate differentiation and for its normal functions. Significantly, the consistent expression of AR in PCa, and its continued activity in PCa that relapse after androgen deprivation therapy (CRPC), indicate that at least a subset of these genes are also critical for PCa development and progression. However, the identity of the AR regulated genes that are critical for PCa remain unclear, and the extent to which AR acquires new functions during PCa development and progression remains to be determined.
AR-INDUCED GENES MEDIATING PCa GROWTH
Consistent with the normal function of androgens in prostate being to drive the differentiated functions of luminal epithelial cells, AR induces many genes coding for seminal fluid proteins (such as prostate-specific antigen) and multiple genes in metabolic pathways required to support high-levels of protein and lipid synthesis.1,2 Significantly, AR does not stimulate the proliferation of normal prostate luminal epithelial cells. However, AR can clearly stimulate PCa growth and androgen deprivation in PCa cell lines causes a G0/G1-cell cycle arrest.3,4 Amongst genes that regulate cell cycle, the AR binds to a site on the cyclin-dependent kinase inhibitor p21 gene and directly increases p21 transcription and protein expression.5 In some contexts, p21 may stimulate cell cycle progression by increasing assembly of cyclin D/CDK4 complexes, which may be a mechanism that contributes to androgen-stimulated PCa growth.
There are data suggesting direct AR regulation of other cell cycle genes, but most genes driving cell cycle progression in response to androgen do not appear to be directly regulated by AR.4 One indirect mechanism mediating proliferation in response to androgens is an increase in TORC1 activity, with a subsequent TORC1 mediated increase in the translation of D-cyclins.2 The increase in TORC1 activity in response to androgen likely reflects the ability of AR to stimulate cellular metabolism by increasing the expression of multiple membrane transporters and other genes driving lipid and protein synthesis.
Androgen stimulation also promotes rapid degradation of the cyclin-dependent kinase inhibitor p27.3,6 Our recent data indicate that this p27 degradation is due to androgen stimulation of TORC2, with the subsequent phosphorylation and activation of AKT and phosphorylation of p27 by AKT at a site that enhances p27 degradation (threonine 157).7 The androgen-mediated stimulation of TORC2 appears to be independent of transcription, but its mechanism remains to be determined. Interestingly, this AKT site on human p27 is not conserved in the mouse, and our recent study using a tetracycline inducible myristoylated-AKT indicates that AKT driven proliferation in mouse prostate epithelium is independent of p27 degradation.8 These observations suggest that studies in mouse models may underestimate the oncogenic activity of PI3 kinase/AKT pathway activation.
Finally, it should be noted that AR is also weakly expressed by subsets of cells in the prostate stroma, and that AR in these cells can stimulate the expression of growth factors such as keratinocyte growth factor/fibroblast growth factor 7.9 Through this mechanism, AR in stromal cells may indirectly regulate growth of the epithelium, and loss of these stromal factors likely contributes to prostate involution after castration.
AR-REPRESSED GENES MEDIATING ANDROGEN SIGNALING AND DNA SYNTHESIS
The mechanisms through which AR functions as a transcriptional activator have been extensively characterized. However, androgens also decrease the expression of multiple genes through direct or indirect mechanisms. One indirect mechanism is by binding to and interfering with other transcription factors such as SP1, which can suppress SP1-mediated transactivation of genes including luteinizing hormone and c-MET.10,11 Other transcription factors that may be similarly antagonized include RUNX2, JUN and SMAD3.12 AR may also suppress the activity of TCF transcription factors by binding to and sequestering nuclear β–catenin.13,14,15,16,17,18
The AR also has been reported to recruit certain transcription corepressors such as ALIEN, DAX1, HEY and AES, but their roles in AR regulation of specific genes remain to be determined.19,20,21,22,23,24 The corepressors NCoR and SMRT associate strongly with the LBD of unliganded nonsteroid nuclear receptors and mediate their transcriptional repression functions. In contrast, unliganded steroid receptors are not tightly associated with chromatin and the roles of NCoR and SMRT are less clear. However, the agonist-liganded AR can associate weakly with NCoR and SMRT, probably through the AR N-terminal domain, and this interaction can modestly suppress AR transcriptional activity.25,26,27,28 Significantly, certain AR antagonists can enhance NCoR and SMRT binding to AR, which may contribute to their activities. Finally, androgen-mediated transcriptional repression has been linked to AR recruitment of EZH2 and an increase in the EZH2 catalyzed repressive H3K27me3 mark.29,30
We recently explored the mechanism through which androgens can suppress AR mRNA levels, and found that the agonist-liganded AR was functioning directly on the AR gene to repress its transcription.31 This repression was mediated through an AR binding site in the second intron of the AR gene, and was dependent on the binding of a histone demethylase, lysine specific demethylase 1 (LSD1, KDM1A) to this site. LSD1 has been extensively characterized as a transcriptional repressor that functions by demethylation of the H3K4me1 and H3K4me2 histone marks associated with enhancers (due to its catalytic mechanism, LSD1 cannot demethylate trimethylated lysines).32 LSD1 associates with the protein CoREST in a repressive complex that also includes histone deactylases (HDAC1 and HDAC2), providing a further mechanism for transcriptional repression.
In addition to the AR gene, we found that AR could similarly repress the expression of genes mediating androgen synthesis (AKR1C3 and HSD17B6), consistent with a negative feedback pathway to regulate AR signaling. Significantly, androgen repressed genes were also highly enriched for genes that are required for DNA synthesis.31 This result is consistent with a normal physiological role of AR being to drive differentiation rather than proliferation. The androgen repression of these genes is presumably overridden in PCa by other oncogenic signal transduction pathways. However, an unintended consequence of androgen deprivation therapy may be to relieve repression of these genes and thereby provide a stimulus for proliferation that may contribute to eventual relapse. If possible, approaches that can selectively block AR activity on AR-stimulated genes, while maintaining or enhancing AR repression of genes mediating DNA synthesis may be more effective than current androgen deprivation therapies.
AR ACQUIRES NEW FUNCTIONS IN TMPRSS2:ERG FUSION POSITIVE TUMORS
Fusions between the strongly AR regulated TMPRSS2 gene and the Ets family transcription factor ERG gene, as well as additional fusions involving TMPRSS2 or other AR regulated genes, have established a genetic mechanism through which AR acquires new functions during PCa development.33 Several genes that may be directly regulated by ERG have been identified, but the precise mechanisms through which ERG drives PCa development have not been clear.34,35
We reported recently that ERG binds to a site downstream of the SOX9 gene in human PCa cells and thereby opens a binding site for AR that is not present in the absence of ERG.36 This site then functions as an AR regulated enhancer resulting in robust androgen stimulated induction of SOX9 (Figure 1). ERG similarly opens cryptic AR regulated enhancers in multiple other genes, but SOX9 appears to be the major effector of ERG in human PCa cells. In particular, SOX9 has been shown to regulate ductal morphogenesis in fetal prostate and to maintain stem/progenitor cells in adult tissues.37,38,39,40 SOX9 overexpression in human PCa cells enhances their growth and invasion, while SOX9 knockdown suppresses their growth.36,40 In mouse models, SOX9 overexpression in prostate on a Pten−/+ background results in high grade dysplastic lesions that can progress to invasive PCa, while SOX9 knockdown can impair PCa development driven by MYC and SV40 T antigen.36,37,41
Figure 1.
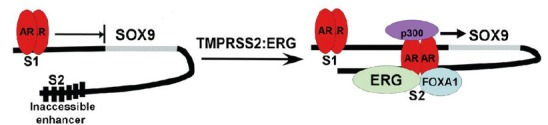
ERG opens cryptic AR regulated enhancer in human SOX9 gene. In the absence of ERG, AR binds weakly to a site upstream of the SOX9 gene (S1 site) and weakly suppresses SOX9 expression. In TMPRSS2:ERG fusion positive tumors, ERG binds to a site downstream of the SOX9 gene (S2 site). It then functions in conjunction with FOXA1 as a pioneer transcription factor to open the site and reveal a cryptic AR-binding site, converting the site into an AR-regulated enhancer. The S1 site is highly conserved across species including mouse, but the S2 site is not conserved. AR: androgen receptor.
A recent study showed that ERG expression in mouse prostate, similarly to ERG in human PCa cells, reprograms AR to stimulate the expression of multiple new genes.42 However, the ERG and AR-binding site identified at the 3’ end of the human SOX9 gene is not conserved in mouse, so that ERG overexpression in mouse prostate does not increase SOX9.36 This may account for the weaker phenotype of transgenic ERG versus transgenic SOX9 overexpression in mouse prostate. Interestingly, a recent study in mouse found that the transcriptional repressor Zbtb7a, which behaves as a tumor suppressor in mouse PCa models, functions by repressing SOX9 transcriptional activity.43 Therefore, while ERG does not directly increase SOX9 expression in mouse, it remains possible that it modulates SOX9 activity or downstream functions. In any case, identification of the critical functions downstream of SOX9 that may drive PCa is now a focus of investigation.
FURTHER NOVEL AR FUNCTIONS ACQUIRED DURING PCa DEVELOPMENT OR PROGRESSION
The spectrum of genes regulated by AR during PCa development or progression may also be altered by epigenetic mechanisms. In a CRPC cell line derived from LNCaP cells (LNCaP-abl), AR was found to have a distinct transcriptional program that included the direct activation of M-phase cell cycle genes such as CDK1 and UBE2C.44 This reprogramming was associated with increased H3K4 methylation and increased AR binding to sites in these genes. These findings presumably reflect selective pressure for tumor cells that have epigenetically silenced AR-regulated genes and opened AR-regulated enhancers controlling genes that drive proliferation. Mutations in FOXA1 and in genes controlling histone methylation being found in advanced PCa could possibly contribute to AR reprogramming.
EZH2 is one such histone methyltransferase that is upregulated in CRPC, and has been well characterized as a component of the polycomb repressive complex 2 that silences genes through H3K27 trimethylation. However, EZH2 also has been identified as an AR coactivator that may contribute to altering AR function in CRPC. A recent study found that EZH2 forms a polycomb repressive complex 2 independent complex with AR in CRPC cells, which is recruited to the cis-regulatory elements of AR target genes including CDK1 and UBE2C.45 Moreover, EZH2 was found to function as an AR coactivator on these genes by a mechanism that is dependent on its methyltransferase activity, but independent of its ability to methylate H3K27. Finally, this AR interaction and coactivator function of EZH2 may be mediated by AKT dependent phosphorylation. As AKT is generally activated in advanced PCa due to PTEN loss, this may be a major mechanism contributing to AR reprogramming.
Interestingly, AR splice variants lacking the LBD, which are increased in CRPC, may also regulate a distinct set of genes that include genes driving cell cycle progression.46 The basis for these differences remains to be determined, but could reflect novel interactions between AR splice variants and coactivators including EZH2. Finally, it should be emphasized that most of our current detailed data on AR regulated genes is derived from studies in model systems. Therefore, despite the challenges, it will be important to translate these findings into clinical samples. Indeed, one recent AR ChIP-seq study in human CRPC samples tumors found evidence of a transcriptional program that was distinct from that found in PCa cell line models.47
COMPETING INTERESTS
The authors declare that they have no competing interests.
ACKNOWLEDGMENTS
This study was supported by grants from the National Institutes of Health, the Department of Defense Prostate Cancer Research Program and awards from the Prostate Cancer Foundation.
REFERENCES
- 1.Massie CE, Lynch A, Ramos-Montoya A, Boren J, Stark R, et al. The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO J. 2011;30:2719–33. doi: 10.1038/emboj.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu Y, Chen SY, Ross KN, Balk SP. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res. 2006;66:7783–92. doi: 10.1158/0008-5472.CAN-05-4472. [DOI] [PubMed] [Google Scholar]
- 3.Knudsen KE, Arden KC, Cavenee WK. Multiple G1 regulatory elements control the androgen-dependent proliferation of prostatic carcinoma cells. J Biol Chem. 1998;273:20213–22. doi: 10.1074/jbc.273.32.20213. [DOI] [PubMed] [Google Scholar]
- 4.Balk SP, Knudsen KE. AR, the cell cycle, and prostate cancer. Nucl Recept Signal. 2008;6:e001. doi: 10.1621/nrs.06001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu S, Liu M, Epner DE, Tsai SY, Tsai MJ. Androgen regulation of the cyclin-dependent kinase inhibitor p21 gene through an androgen response element in the proximal promoter. Mol Endocrinol. 1999;13:376–84. doi: 10.1210/mend.13.3.0254. [DOI] [PubMed] [Google Scholar]
- 6.Lu L, Schulz H, Wolf DA. The F-box protein SKP2 mediates androgen control of p27 stability in LNCaP human prostate cancer cells. BMC Cell Biol. 2002;3:22. doi: 10.1186/1471-2121-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang Z, Zhang T, Dizeyi N, Chen S, Wang H, et al. Androgen Receptor Enhances p27 Degradation in Prostate Cancer Cells through Rapid and Selective TORC2 Activation. J Biol Chem. 2012;287:2090–8. doi: 10.1074/jbc.M111.323303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Xu Y, Fang Z, Chen S, Balk SP, et al. Doxycycline regulated induction of AKT in murine prostate drives proliferation independently of p27 cyclin dependent kinase inhibitor downregulation. PLoS One. 2012;7:e41330. doi: 10.1371/journal.pone.0041330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan G, Fukabori Y, Nikolaropoulos S, Wang F, McKeehan WL. Heparin-binding keratinocyte growth factor is a candidate stromal-to-epithelial-cell andromedin. Mol Endocrinol. 1992;6:2123–8. doi: 10.1210/mend.6.12.1491693. [DOI] [PubMed] [Google Scholar]
- 10.Curtin D, Jenkins S, Farmer N, Anderson AC, Haisenleder DJ, et al. Androgen suppression of GnRH-stimulated rat LHbeta gene transcription occurs through Sp1 sites in the distal GnRH-responsive promoter region. Mol Endocrinol. 2001;15:1906–17. doi: 10.1210/mend.15.11.0723. [DOI] [PubMed] [Google Scholar]
- 11.Verras M, Lee J, Xue H, Li TH, Wang Y, et al. The androgen receptor negatively regulates the expression of c-Met: implications for a novel mechanism of prostate cancer progression. Cancer Res. 2007;67:967–75. doi: 10.1158/0008-5472.CAN-06-3552. [DOI] [PubMed] [Google Scholar]
- 12.Grosse A, Bartsch S, Baniahmad A. Androgen receptor-mediated gene repression. Mol Cell Endocrinol. 2012;352:46–56. doi: 10.1016/j.mce.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 13.Truica CI, Byers S, Gelmann EP. Beta-catenin affects androgen receptor transcriptional activity and ligand specificity. Cancer Res. 2000;60:4709–13. [PubMed] [Google Scholar]
- 14.Shah S, Hecht A, Pestell R, Byers SW. Trans-repression of beta-catenin activity by nuclear receptors. J Biol Chem. 2003;278:48137–45. doi: 10.1074/jbc.M307154200. [DOI] [PubMed] [Google Scholar]
- 15.Yang F, Li X, Sharma M, Sasaki CY, Longo DL, et al. Linking beta-catenin to androgen-signaling pathway. J Biol Chem. 2002;277:11336–44. doi: 10.1074/jbc.M111962200. [DOI] [PubMed] [Google Scholar]
- 16.Chesire DR, Isaacs WB. Ligand-dependent inhibition of beta-catenin/TCF signaling by androgen receptor. Oncogene. 2002;21:8453–69. doi: 10.1038/sj.onc.1206049. [DOI] [PubMed] [Google Scholar]
- 17.Mulholland DJ, Read JT, Rennie PS, Cox ME, Nelson CC. Functional localization and competition between the androgen receptor and T-cell factor for nuclear beta-catenin: a means for inhibition of the Tcf signaling axis. Oncogene. 2003;22:5602–13. doi: 10.1038/sj.onc.1206802. [DOI] [PubMed] [Google Scholar]
- 18.Chen SY, Wulf G, Zhou XZ, Rubin MA, Lu KP, et al. Activation of beta-catenin signaling in prostate cancer by peptidyl-prolyl isomerase Pin1-mediated abrogation of the androgen receptor-beta-catenin interaction. Mol Cell Biol. 2006;26:929–39. doi: 10.1128/MCB.26.3.929-939.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan X, Lu ML, Li T, Balk SP. SRY interacts with and negatively regulates androgen receptor transcriptional activity. J Biol Chem. 2001;276:46647–54. doi: 10.1074/jbc.M108404200. [DOI] [PubMed] [Google Scholar]
- 20.Jouravel N, Sablin E, Arnold LA, Guy RK, Fletterick RJ. Interaction between the androgen receptor and a segment of its corepressor SHP. Acta Crystallogr D Biol Crystallogr. 2007;63:1198–200. doi: 10.1107/S0907444907045702. [DOI] [PubMed] [Google Scholar]
- 21.Moehren U, Papaioannou M, Reeb CA, Hong W, Baniahmad A. Alien interacts with the human androgen receptor and inhibits prostate cancer cell growth. Mol Endocrinol. 2007;21:1039–48. doi: 10.1210/me.2006-0468. [DOI] [PubMed] [Google Scholar]
- 22.Gamble SC, Chotai D, Odontiadis M, Dart DA, Brooke GN, et al. Prohibitin, a protein downregulated by androgens, represses androgen receptor activity. Oncogene. 2007;26:1757–68. doi: 10.1038/sj.onc.1209967. [DOI] [PubMed] [Google Scholar]
- 23.Belandia B, Powell SM, Garcia-Pedrero JM, Walker MM, Bevan CL, et al. Hey1, a mediator of notch signaling, is an androgen receptor corepressor. Mol Cell Biol. 2005;25:1425–36. doi: 10.1128/MCB.25.4.1425-1436.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu X, Li P, Roeder RG, Wang Z. Inhibition of androgen receptor-mediated transcription by amino-terminal enhancer of split. Mol Cell Biol. 2001;21:4614–25. doi: 10.1128/MCB.21.14.4614-4625.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoon HG, Wong J. The corepressors silencing mediator of retinoid and thyroid hormone receptor and nuclear receptor corepressor are involved in agonist-and antagonist-regulated transcription by androgen receptor. Mol Endocrinol. 2006;20:1048–60. doi: 10.1210/me.2005-0324. [DOI] [PubMed] [Google Scholar]
- 26.Cheng S, Brzostek S, Lee SR, Hollenberg AN, Balk SP. Inhibition of the dihydrotestosterone-activated androgen receptor by nuclear receptor corepressor. Mol Endocrinol. 2002;16:1492–501. doi: 10.1210/mend.16.7.0870. [DOI] [PubMed] [Google Scholar]
- 27.Hodgson MC, Astapova I, Cheng S, Lee LJ, Verhoeven MC, et al. The androgen receptor recruits nuclear receptor CoRepressor (N-CoR) in the presence of mifepristone via its N and C termini revealing a novel molecular mechanism for androgen receptor antagonists. J Biol Chem. 2005;280:6511–9. doi: 10.1074/jbc.M408972200. [DOI] [PubMed] [Google Scholar]
- 28.Song LN, Coghlan M, Gelmann EP. Antiandrogen effects of mifepristone on coactivator and corepressor interactions with the androgen receptor. Mol Endocrinol. 2004;18:70–85. doi: 10.1210/me.2003-0189. [DOI] [PubMed] [Google Scholar]
- 29.Zhao JC, Yu J, Runkle C, Wu L, Hu M, et al. Cooperation between Polycomb and androgen receptor during oncogenic transformation. Genome Res. 2012;22:322–31. doi: 10.1101/gr.131508.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chng KR, Chang CW, Tan SK, Yang C, Hong SZ, et al. A transcriptional repressor co-regulatory network governing androgen response in prostate cancers. EMBO J. 2012;31:2810–23. doi: 10.1038/emboj.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai C, He HH, Chen S, Coleman I, Wang H, et al. Androgen receptor gene expression in prostate cancer is directly suppressed by the androgen receptor through recruitment of lysine-specific demethylase 1. Cancer Cell. 2011;20:457–71. doi: 10.1016/j.ccr.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–53. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–8. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 34.Tomlins SA, Laxman B, Varambally S, Cao X, Yu J, et al. Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia. 2008;10:177–88. doi: 10.1593/neo.07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu J, Yu J, Mani RS, Cao Q, Brenner CJ, et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell. 2010;17:443–54. doi: 10.1016/j.ccr.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cai C, Wang H, He HH, Chen S, He L, et al. ERG induces androgen receptor-mediated regulation of SOX9 in prostate cancer. J Clin Invest. 2013;123:1109–22. doi: 10.1172/JCI66666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang Z, Hurley PJ, Simons BW, Marchionni L, Berman DM, et al. Sox9 is required for prostate development and prostate cancer initiation. Oncotarget. 2012;3:651–63. doi: 10.18632/oncotarget.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schaeffer EM, Marchionni L, Huang Z, Simons B, Blackman A, et al. Androgen-induced programs for prostate epithelial growth and invasion arise in embryogenesis and are reactivated in cancer. Oncogene. 2008;27:7180–91. doi: 10.1038/onc.2008.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomsen MK, Butler CM, Shen MM, Swain A. Sox9 is required for prostate development. Dev Biol. 2008;316:302–11. doi: 10.1016/j.ydbio.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Leav I, Ibaragi S, Wegner M, Hu GF, et al. SOX9 is expressed in human fetal prostate epithelium and enhances prostate cancer invasion. Cancer Res. 2008;68:1625–30. doi: 10.1158/0008-5472.CAN-07-5915. [DOI] [PubMed] [Google Scholar]
- 41.Thomsen MK, Ambroisine L, Wynn S, Cheah KS, Foster CS, et al. Transatlantic Prostate Group. SOX9 elevation in the prostate promotes proliferation and cooperates with PTEN loss to drive tumor formation. Cancer Res. 2010;70:979–87. doi: 10.1158/0008-5472.CAN-09-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Y, Chi P, Rockowitz S, Iaquinta PJ, Shamu T, et al. ETS factors reprogram the androgen receptor cistrome and prime prostate tumorigenesis in response to PTEN loss. Nat Med. 2013;19:1023–9. doi: 10.1038/nm.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang G, Lunardi A, Zhang J, Chen Z, Ala U, et al. Zbtb7a suppresses prostate cancer through repression of a Sox9-dependent pathway for cellular senescence bypass and tumor invasion. Nat Genet. 2013;45:739–46. doi: 10.1038/ng.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Q, Li W, Zhang Y, Yuan X, Xu K, et al. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell. 2009;138:245–56. doi: 10.1016/j.cell.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu K, Wu ZJ, Groner AC, He HH, Cai C, et al. EZH2 oncogenic activity in castration-resistant prostate cancer cells is Polycomb-independent. Science. 2012;338:1465–9. doi: 10.1126/science.1227604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu R, Lu C, Mostaghel EA, Yegnasubramanian S, Gurel M, et al. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res. 2012;72:3457–62. doi: 10.1158/0008-5472.CAN-11-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma NL, Massie CE, Ramos-Montoya A, Zecchini V, Scott HE, et al. The androgen receptor induces a distinct transcriptional program in castration-resistant prostate cancer in man. Cancer Cell. 2013;23:35–47. doi: 10.1016/j.ccr.2012.11.010. [DOI] [PubMed] [Google Scholar]


