Fig. 2.
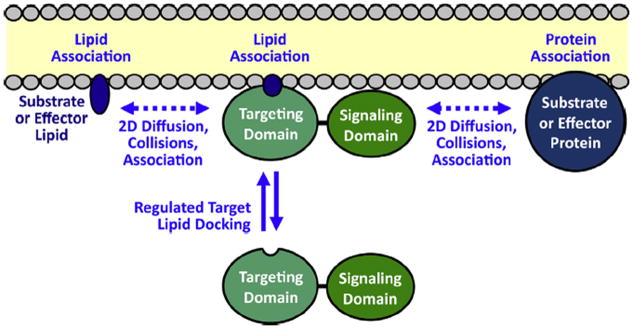
Function of PH and C2 membrane targeting domains in the recruitment and activation of signaling proteins on target membrane surfaces (Corbalan-Garcia et al., 2007; Leonard and Hurley, 2011; Newton, 2009; Lemmon, 2008; Cho, 2001; Nalefski and Falke, 1996). First, a 2° messenger signal triggers the recruitment of the signaling protein targeting domain from the cytoplasm to the bilayer surface: many PH domains are recruited to the plasma membrane by a PI(3,4,5)P3 (PIP3) lipid signal, while the C2 domains of classical protein kinase C isoforms (cPKCs) are activated by Ca2+ binding and then dock to PS and PI(4,5)P2 (PIP2) on the plasma membrane. Second, the signaling domain of each recruited protein is activated by its proximity on the membrane surface to membrane-associated protein and/or lipid molecules that serve as effectors or substrates. At the leading edge membrane, PIP3 and Ca2+ both recruit signaling proteins and establish a signaling network on the bilayer surface. Lateral, 2-dimensional diffusion on the bilayer is required for the signaling proteins to encounter and bind their signaling partners, and for the resulting membrane-bound products to diffuse away. Thus, the lipid bilayer serves as a 2-dimensional platform for the assembly of the dynamic signaling circuit, and for the diffusion of its many components during signaling reactions.
