Introduction
Co-activation is the simultaneous activation of agonist and antagonist muscle groups around a joint which contributes to joint stability, homogeneous load distribution [Baratta et al, 1988], control of bone displacements [Solomonow et al, 1987] and movement efficiency [Levine et al, 1952]. Co-activation of knee joint muscles has been extensively studied over the past two decades due to its importance during ambulation and balance [Baratta et al, 1988; Seyedali et al, 2012]. Opposing muscle groups such as the quadriceps and the hamstrings function as synergists to provide stability and stiffness to the knee joint [Ait-Haddou et al, 2000]. Selected joint pathologies, central or peripheral nervous system disorders can induce abnormal levels of co-activation [Busse et al, 2005]. Inappropriate co-activation levels produce movement dysfunction, which in turn can lead to joint injury [Baratta et al, 1988; Busse et al., 2005; Macaluso et al, 2002]. Reliable and meaningful measures are needed that accurately assess co-activation levels by calculation of the co-activation index (CI). Such a CI will permit comparisons between studies and serve as an outcome measure for rehabilitation interventions.
There are a number of parameters that may affect the reliability and validity of the CI calculation. Parameters that are related to the data collection include the number of muscles or muscle segments sampled, pennation angle, the inclusion of monoarticular or multiarticular muscles, type of contraction, joint position, and electrode placement. Parameters that are related to data analysis include the selection of the time unit (window) and the smoothing approach applied to the electromyographic (EMG) signal, as well as the equation/method for the quantification of the CI. While most data collection parameters have inherent and inevitable limitations that affect comparison among studies, parameters that are related to data analysis can be controlled and standardized.
There are four commonly utilized methods for the quantification of the CI. The first two rudimentary methods were the semi-quantitative estimates of EMG magnitude [Frost et al, 1997] and the agonist-to-antagonist ratio of EMG activity utilizing millivolts of electrical activity [Damiano et al, 2000; Fung et al, 1989]. The limitations of these two methods led to the adoption of more robust techniques that normalized the EMG amplitude for each of the agonist and antagonist muscle groups to the respective maximum voluntary contraction values (MVC; [Ervilha et al, 2012; Knutson et al, 1994]). The last and more recent method for the calculation of the CI quantified the antagonist moment using mathematical modeling of the EMG/joint torque relationship, but with controversial applicability due to changes in the slope attributable to evolution of the firing frequency and recruitment across the range of muscle activation [Merletti et al, 2004].
Normalization methods have been widely adopted but there are many inconsistencies with respect to window size and smoothing techniques utilized to estimate muscle activation. These inconsistencies reduce the comparability of calculated CIs between studies. Researchers have used peak EMG amplitude [Yang et al, 1984], average EMG [Kellis et al, 2011], integrated EMG [Kubo et al, 2004], root mean square [Hortobágyi et al, 2005] and envelope EMG [Frost et al, 1997] of various window sizes among other filtering and smoothing techniques. Besides the peak amplitude technique, which estimates muscle activation from a single value, the other techniques calculate an average value over a selected segment of data (window). Signal processing using RMS requires fewer steps in the data reduction process and minimizes signal distortion [Cram et al, 1998]. The second important issue is the selection of the optimum window size. Utilizing a small window or even choosing a single value (e.g., peak amplitude) can be affected by artifacts or outliers. A larger EMG window that is temporally associated with the highest joint torque produced during the MVC may be more representative of the muscle’s activation. On the contrary, an excessively large window size may distort estimates by including segments of submaximal muscle activation. It still remains unanswered which data smoothing method and window size can generate the most reliable and meaningful CI.
Replication of electrode placement can be a limiting factor in between-day reproducibility. Electrode placement on the belly of an agonistic muscle during MVC has produced very reliable between-day estimates of maximal muscle EMG [Larsson et al, 2003; McKenzie et al, 2010]. However, when assessing co-activation, the antagonist muscle group undergoes a submaximal contraction. During submaximal contractions, a slight shift in electrode placement between sessions could capture different EMG activity or increase the variability of the signal [Van Dijk et al, 2009] due to changes in spatial summation of the signals.
Therefore, the purpose of the present study was to assess thigh muscle CI during isometric contractions by comparing the results from commonly employed signal processing techniques and to determine within- and between-session CI reliability. It was hypothesized that RMS EMG of a window size around the peak torque during a maximum voluntary isometric contraction would produce more reliable estimates of CI. Additionally, we hypothesized that within-session reliability would be higher than between-session values.
Methods
Informed consent
The study was approved by the Creighton University Institutional Review Board and conducted in accordance with the Declaration of Helsinki. All subject volunteers read, understood and signed the informed consent document prior to participation.
Subjects
Ten healthy young adults (5 males, 5 females; 27.5±4yrs, 174.6±12cm; 77.8±12kg) volunteered from a convenience sample of young, healthy university students that were enrolled in health sciences graduate curricula. None of the volunteers were currently participating in formal collegiate sports teams. To minimize practice/learning effects, subjects were required to have prior experience with an isokinetic dynamometer. Subjects were free of musculoskeletal and/or neurological problems that may have affected their ability to generate maximal knee flexion and extension torque in the dominant (preferred kicking) leg.
Torque recordings
Subjects were positioned and restrained on a Biodex System 3 isokinetic dynamometer per manufacturer recommendations for knee assessment (Biodex Medical Systems Inc., Shirley, NY, USA) with joint angles of 60° at both the hip and knee using zero-neutral measurements. The lateral epicondyle was aligned with the dynamometer’s power shaft and the inferior edge of the ankle cuff was placed 2 cm above the lateral malleolus. The subject’s arms were kept folded in front of their chest during testing. The Biodex raw torque signal was digitally sampled at 1 kHz and stored on a PC (Windows XP) running DataPac 2K2 (Run Technologies, Mission Viejo, CA, USA). The raw torque data were low pass filtered at 10Hz, converted to Newton-meters and corrected for gravity offset.
EMG recordings
The skin over the vastus lateralis, rectus femoris, vastus medialis, lateral and medial hamstrings was shaved and wiped with alcohol on the dominant side. Pairs of surface EMG electrodes (BIOPAC Systems, Inc., Goleta, CA, USA) were attached over each muscle following the SENIAM guidelines maintaining a 2 cm center-to-center interelectrode spacing [Hermens et al, 2000]. A ground electrode was placed on the ipsilateral lateral malleolus. Electrodes were attached by short, shielded wires to on-site preamplifiers. The differential amplifiers had a gain of 1000–2000, an input impedance of 100kMΩ, and a common mode rejection ratio of 100dB (Motion Lab Systems MA-300™). EMG signals and the Biodex torque signal were digitally sampled at 1 kHz per and stored on a PC using DataPac 2K2. Using a custom-written Matlab program (Matlab 2012b; Mathworks Inc., Natick, MA, USA), EMG signals were band-pass filtered (10 to 450 Hz), notch filtered at 60 Hz, corrected for zero offset, full-wave rectified and stored for further analysis.
Experimental design
After measuring and recording stature and body mass, each subject warmed-up by walking on a treadmill for 5 min at 5–6 km/h. Prior to testing, each subject was allowed to become accustomed to the dynamometer by performing brief submaximal and maximal contractions for both knee extension and flexion.. Three to four familiarization contractions lasting 2–3 sec were allowed in each direction. A computer monitor provided feedback of their performance by displaying the torque curve in real time while a horizontal line demarcated the subject’s highest torque.
In the first session, each subject performed one knee flexion MVIC, rested for two minutes then four successive maximum knee extension MVICs. They were instructed to flex or extend the knee as hard as possible for 2–3 seconds. One minute of rest was allowed between each extension contraction. Upon successful completion of the first session, all subjects were retested with a minimum of 3 days between sessions.
Data processing
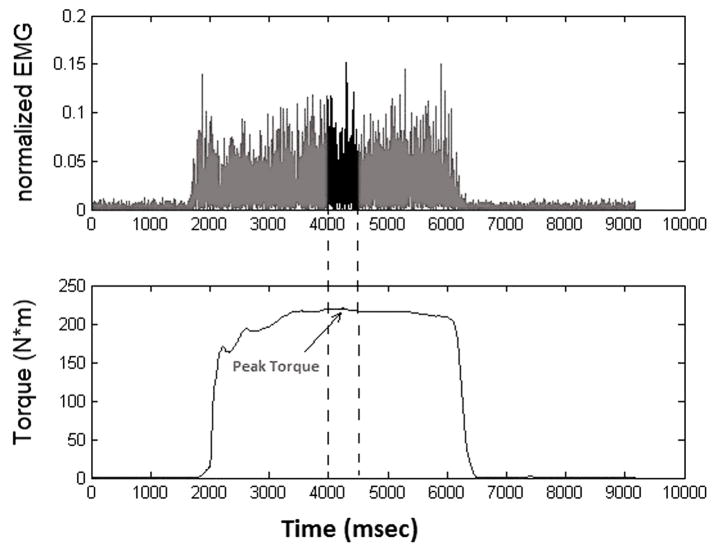
EMG activity was used to quantify CI during quadriceps maximum voluntary isometric contraction (MVIC). The CI was calculated using 7 different approaches that were based on a) a single value (the peak amplitude of the raw EMG signal), b) an interval around the peak torque [20msec (RMS20PT), 50msec (RMS50PT) and 500msec (RMS500PT); Figure 1], and c) throughout the entire period of extensor torque production [for the entire burst (RMSBURST), and in moving windows of 20 (RMS20) and 50msec (RMS50)]. Except for the single value method, the EMG activity of each muscle segment was normalized to the corresponding MVIC. Equation 1 provides the CI calculation where the numerator and the denominator represent the corresponding values from any of the measured parameters for each of the 7 approaches. The CI was calculated for each of the four knee extension MVICs.
Figure 1.
The top portion of the figure displays the normalized activation from one of the lateral hamstring muscle group. In this example, the Matlab algorithm calculated the RMS in a window of 500ms around the peak torque, which is one of the 7 analytical approaches utilized for the quantification of the CI.
| (1) |
Statistical analysis was performed using SPSS software (SPSS 20; SPSS Inc., Chicago, IL, USA). Descriptive statistics including means, standard deviations (SD) and coefficient of variation (CV) were calculated for each parameter. A paired t-test was performed between sessions to test for learning effect. Intraclass correlation coefficient (ICC) analysis was used to assess the consistency, or conformity, of measurements made by applying the different calculation approaches. According to Sleivert and Wenger [1994], ICC values below 0.6 indicates poor reproducibility, while it has been suggested that ICC values greater than 0.8 are acceptable for clinical work [Currier, 1984]. To give more practical meaning to the ICC measurements, minimum detectable change (MDC) was calculated by multiplying the standard error of measurement (SEM) by 2.77 [Weir, 2005]. MDC indicates the level of change in a parameter attributed with 95% certainty to a true change in a subject’s condition, as differentiated from changes due to test-retest errors. In other words, any retest measurement should exceed the MDC to indicate a real change. In addition, inter-session CV and intra-session CV values were calculated to determine between- and within-sessions variability, respectively.
Results
CI values ranged from 11.28 to 13.17. The calculations employing peak amplitude across the entire burst gave the highest CI values and methods using RMS around the PT produced the lowest CI values (Table 1). A paired t-test revealed no statistical difference in CI between sessions therefore mean CI was calculatedfor each subject by taking the average of all contractions across sessions.
Table 1.
Within- and between-session variability for the different quantification methods of the coactivation index.
| CI (mean±SD) | Within-session | Between-session | |
|---|---|---|---|
|
| |||
| CV (%) | CV (%) | ||
| Amplitude | 13.17 (8.2) | 14.2% | 34.0% |
| RMS20PT | 11.54 (7.4) | 21.8% | 30.2% |
| RMS50PT | 11.50 (7.6) | 19.9% | 30.3% |
| RMS500PT | 11.28 (7.8) | 10.5% | 24.9% |
| RMS20 | 12.68 (8.5) | 13.8% | 31.9% |
| RMS50 | 12.78 (9.1) | 12.6% | 31.1% |
| RMSBURST | 11.64 (7.6) | 9.0% | 24.2% |
CI = Coactivation Index; CV = Coefficient of Variation.
Within-session
ICC analysis of the CI quantification approaches revealed that within-session coefficients were greater than 0.80 with most values greater than 0.90, while MDC values ranged from 3.5% to 7.7% (Table 2). The low MDC value with the 500ms-window approach indicated that changes exceeding 3.7 units of the CI were beyond measurement error. CV values ranged from 9.0% to 21.8% (Table 1). Within-session results indicated that CI was highly reliable for all approaches. The 500ms-window and the entire burst window yielded the most reliable and the least variable CI.
Table 2.
Within- and between-session reliability indices for the different quantification methods of the coactivation index.
| Within-session | Between-session | |||
|---|---|---|---|---|
|
| ||||
| ICC | MDC | ICC | MDC | |
| Amplitude | 0.910 | 6.7 | 0.461 | 14.1 |
| RMS20PT | 0.861 | 7.3 | 0.578 | 11.7 |
| RMS50PT | 0.896 | 6.6 | 0.645 | 11.4 |
| RMS500PT | 0.971 | 3.7 | 0.664 | 11.4 |
| RMS20 | 0.900 | 7.2 | 0.431 | 15.0 |
| RMS50 | 0.904 | 7.7 | 0.376 | 16.6 |
| RMSBURST | 0.979 | 3.5 | 0.680 | 10.9 |
MDC = Minimum Detectable Change; ICC = Intraclass Correlation Coefficient.
Between-session
ICC values for the between-session analysis ranged from 0.376 to 0.680, while MDC ranged from 11.9% to 20% (Table 2). CV values ranged from 24.2% to 34% (Table 1). Between-session ICC values were consistently lower than within-session ICC values across all CI approaches. The opposite holds true for CV values with between-session values consistently higher than within-session CV values.
Discussion
The primary goal of the present study was to determine within- and between-session CI reliability from commonly employed signal processing techniques. We found notable differences in the reliability and variability measures among the signal processing techniques, which partially supported our first hypothesis. In support of our second hypothesis, within-session measures of reliability displayed higher reliability and lower variability than between-session measures. Nonetheless, the average value of CI across the different approaches fluctuated between 11.28 to 13.17, which lies within the range reported in the literature [Grabiner et al, 1989].
Within-session measures
Overall, ICC analysis indicated that CI quantification was highly reliable within the same session independent of the approach employed (ICC >0.86). To our knowledge, only one study has reported test-retest measures by calculating reliability coefficients [Falconer et al, 1985]. However, the study by Falconer et al [1985] examined the co-activation of the ankle muscles during gait. As such, interpretation of the ICC of the present study should be restricted to the reliability of CI calculations based on EMG from isometric knee extension contractions, since the CI equation depends solely on the ratio between the EMG activity of the antagonist and agonist muscles (equation 1). It is well established that EMG activity of knee flexors and extensors is highly reliable during contractions close to MVIC (ICC>80; McKenzie et al, 2010; Viitasalo et al, 1975], while there is a moderate to high reliability for MH and LH at intensities 10–30% MVIC (0.73 < ICC < 0.96; Kellis et al, 2008]. The high within-session reliability values in the present study are consistent with these reports.
In support of our first hypothesis, the RMS500PT approach yielded the most reliable and least variable CI values. The other two approaches, which utilized a much smaller window size around the PT, yielded a twofold increase in variability and a proportional increase in the MDC values. The primary reason for this discrepancy was that PT does not necessarily coincide with the highest electrical activation of the muscles involved due to electromechanical delay. Therefore a 20 or 50ms window around PT may not have captured the maximum activity of the EMG signal. Another influential factor was the number of data points analysed. A small window size could be affected more by artifacts or unusual EMG activity due to motion or other electrical interference, whereas a larger window size could be less influenced by such activity. The same phenomenon could influence the other two approaches that utilized a moving window of 20 and 50ms. In addition, the two latter approaches could have been influenced by the fact that antagonist EMG activity was greater at the beginning and end of a movement [Baratta et al, 1988; Kellis, 1998]. The increases in antagonist activity at the beginning and end of contraction would have been accentuated during dynamic or isokinetic contractions in which there was acceleration and deceleration of the limb at the initial and final parts of the movement, respectively [Hagood et al, 1990; Kellis et al, 1996; Osternig et al, 1984]. However, when assessing isometric contractions using the Biodex apparatus, there was a small limb movement that occurred between rest and full exertion due to compression of the cushioning material of the restraining straps and chair. These small movements could have created artifacts or higher activations of the antagonist muscle group.
The RMSBURST approach yielded results that were comparable to the RMS500PT approach. This was expected since the average PT during a MVIC was close to 95% of the knee extensors’ PT. The initial and final parts of the contraction were only a small fraction of the entire burst and may not have affected the calculated outcome. However, when analysing longer contractions that contain submaximal effort or contractions in which the PT lasts only a few milliseconds, selection of an appropriate window size must be carefully considered. Further research is required to investigate the CI calculation algorithms for submaximal muscle contractions similar to the conditions occurring during functional activities and if CI calculations are affected by studying populations with impaired motor control..
Between-session measures
ICC values fluctuated between 0.376 and 0.680 indicating a poor to moderate between-session reliability. In support of our second hypothesis, these values were much lower than the corresponding within-session values. Also, MDC and CV were much higher between- than within-session. The PT normalized to body weight (1.69±0.3) for knee extension was highly reliable within- and between-sessions (ICC 0.982 and 0.958, respectively). Subjects were able to reproduce their PT at both sessions, which implied that any variations in CI values were not due to variations in torque output. The underlying cause of this discrepancy may have been the placement of the electrodes and/or the spatial pattern of motor unit recruitment within the muscle.
Surface electrodes captured the EMG activity of motor units within a limited distance from the skin placement. During a knee extension MVIC – ideally – all or most of the agonist muscle motor units within a detectable range from the pickup electrodes were fully active, thus a slight shift in electrode placement between sessions would not have markedly affected the measured EMG activity. On the contrary, the antagonist muscle group underwent a submaximal contraction and therefore fewer motor units within pickup range were active during an agonist MVIC. The consistency of our torque measurements implied that the net tension generated by the motor units was not altered within the same session. However, poor to moderate between-session reliability in antagonist EMG could be attributed to between-session differences in the subpopulation of recruited motor units and/or small changes in electrode placement that affected the net spatial summation of the motor unit electrical activity within detection range. In addition, reliability outcomes can be affected by the analytical approach employed for the quantification of the CI.
Our review of literature on CI quantification showed that the most commonly employed approaches utilized a moving window of a small size or the peak amplitude of the raw EMG signal. Our current findings clearly showed that a small sampling window elicited the lowest ICC values (0.376 < ICC < 0.441). ICC values lower than 0.6 are considered to have poor reproducibility [Sleivert et al, 1994] and may produce inconclusive or misleading results. However, the RMS500 and RMSBURST approaches generated the highest reliability and lowest variability values, which correspond to moderate reproducibility in general. Nonetheless, these approaches may be reliable and effective as a relative measure among conditions and are best utilized for within-session experimental designs.
Limitations
Potential limitations of our current study were the inherent issues with bipolar surface electrodes, such as cross-talk and placement. Advancement in EMG acquisition systems and proper preparation has minimized cross-talk [Hof, 1984; Winter et al, 1994], while careful measurements following electrode placement standards such as SENIAM can minimize this source of placement error. Other researchers have applied indelible ink skin markings during the first testing to improve accuracy of electrode application during subsequent sessions [Zech et al, 2008]. Nonetheless, neither of the above methods can control for subtle variations in motor unit recruitment patterns, which in turn varies spatial summation of the electrical signal. Perhaps spatial summation variations in submaximal contractions could be addressed by employing high-density surface EMG electrode arrays rather than standard bipolar arrangement [Drost et al, 2006].
Another potential limitation was the difference in the execution strategy between subjects. The knee joint is a complex articular structure controlled by a number of interacting elements including muscles, tendons and ligaments. Subjects could produce similar torque output by using subtly different limb positions and muscle activation patterns. For instance, changes in femoral or tibial rotation along with ankle dorsi/plantar flexion may have affected the recruitment and subsequent EMG motor unit activation of the hamstring and gastrocnemius muscles during a knee extensor’s MVIC. While we were largely able to control the femur and hip joint position, the tibia was able to rotate in the transverse plane and ankle was able to freely move in all planes during the testing procedures. We intentionally focused on well-controlled isometric knee extension contractions to minimize differences in execution strategies. Studies that utilize inherently more variable dynamic contractions impose complex demands such as variations in angular velocity, changes in internal and external torque and muscle length/tension changes. We speculate that CI reliability would decrease for dynamic contractions and would reflect within- and between-subject variations in execution strategy.
Lastly, a potential limitation can be the equation utilized to calculate the CI. Following the overwhelming majority of the current literature, the CI calculation was based on the average EMG activity of three quadriceps and the average EMG activity of two hamstrings muscle segments for a specific time window. However, due to anatomic and fiber-type differences between muscle segments, each segment does not contribute equally to muscle tension. Future studies should focus on weighting the EMG of each segment to account for its overall contribution to forcegeneration, as well as examining the effect of the combination of muscle segments used in the calculation.
Conclusion
Interpretation of the present findings can offer insight into the technique that is more appropriate for the quantification of CI during isometric knee extension at 60° of flexion. A selection of a large window size around the PT appeared to deliver more reliable and less variable results. However, the lower values of between-session reliability accompanied with more than a twofold increase in variability suggests caution in calculation and interpretation of CI values across sessions and between research reports.
Acknowledgments
Research reported in this article was partially supported by the National Institute on Aging of the National Institutes of Health under grant number R15 AG040616.
Biographies

Dimitrios Katsavelis, PhD, is a postdoctoral research fellow in the Department of Physical Therapy at Creighton University. He received an MS in Exercise Science at the University of Nebraska at Omaha and earned his doctoral degree at the University of Nebraska Medical Center with work on biomechanics and motor control during ambulation in a Virtual Reality environment. He is specialized in the area of biomechanics, human anatomy and software engineering. His research interests include motion analysis, muscle activation and torque production characteristics of movement in healthy and pathological populations.

A. Joseph Threlkeld, PT, PhD is an associate professor of Physical Therapy at Creighton University. His physical therapist education and his PhD in anatomy were carried out at the University of Kentucky. Dr. Threlkeld is a founding faculty member of Creighton’s Physical Therapy program. He chaired the Department of Physical Therapy from 1996 to 2001 and is the current director of the Rehabilitation Science Research Laboratory. His research interests focus on the effect of body weight support on human locomotion and the differentiation of central versus peripheral fatigue in people with Parkinson’s disease.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ait-Haddou R, Binding P, Herzog W. Theoretical considerations on cocontraction of sets of agonistic and antagonistic muscles. Journal of Biomechanics. 2000;33(9):1105–11. doi: 10.1016/s0021-9290(00)00085-3. [DOI] [PubMed] [Google Scholar]
- Baratta R, Solomonow M, Zhou BH, Letson D, Chuinard R, D’Ambrosia R. Muscular coactivation. The role of the antagonist musculature in maintaining knee stability. The American Journal of Sports Medicine. 1988;16(2):113–22. doi: 10.1177/036354658801600205. [DOI] [PubMed] [Google Scholar]
- Busse ME, Wiles CM, van Deursen RW. Muscle co-activation in neurological conditions. Physical Therapy Reviews. 2005;10:247–53. [Google Scholar]
- Cram JR, Kasman GS, Holtz J. Introduction to surface electromyography. Gaithersburg: Aspen; 1998. [Google Scholar]
- Currier DP. Elements of research in Physical Therapy. 2. Baltimore: Williams and Wilkins; 1984. [Google Scholar]
- Damiano DL, Martellotta TL, Sullivan DJ, Granata KP, Abel MF. Muscle force production and functional performance in spastic cerebral palsy: relationship of cocontraction. Archives of Physical Medicine and Rehabilitation. 2000;81(7):895–900. doi: 10.1053/apmr.2000.5579. [DOI] [PubMed] [Google Scholar]
- Drost G, Stegeman DF, van Engelen BG, Zwarts MJ. Clinical applications of high-density surface EMG: a systematic review. Journal of Electromyography and Kinesiology. 2006;16(6):586–602. doi: 10.1016/j.jelekin.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Ervilha UF, Graven-Nielsen T, Duarte M. A simple test of muscle coactivation estimation using electromyography. Brazilian Journal of Medical and Biological Research. 2012;45(10):977–81. doi: 10.1590/S0100-879X2012007500092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer K, Winter DA. Quantitative assessment of cocontraction at the ankle joint in walking. Electromyography and Clinical Neurophysiology. 1985;25(2–3):135–49. [PubMed] [Google Scholar]
- Frost G, Dowling J, Dyson K, Bar-Or O. Cocontraction in three age groups of children during treadmill locomotion. Journal of Electromyography and Kinesiology. 1997;7(3):179–86. doi: 10.1016/s1050-6411(97)84626-3. [DOI] [PubMed] [Google Scholar]
- Fung J, Barbeau H. A dynamic EMG profile index to quantify muscular activation disorder in spastic paretic gait. Electromyography and Clinical Neurophysiology. 1989;73(3):233–44. doi: 10.1016/0013-4694(89)90124-7. [DOI] [PubMed] [Google Scholar]
- Grabiner MD, Campbell KR, Hawthorne DL, Hawkins DA. Electromyographic study of the anterior cruciate ligament-hamstring synergy during isometric knee extension. Journal of Orthopaedic Research. 1989;7(1):152–5. doi: 10.1002/jor.1100070122. [DOI] [PubMed] [Google Scholar]
- Hagood S, Solomonow M, Baratta R, Zhou BH, D’Ambrosia The effect of joint velocity on the contribution of the antagonist musculature to knee stiffness and laxity. The American Journal of Sports Medicine. 1990;18(2):182–7. doi: 10.1177/036354659001800212. [DOI] [PubMed] [Google Scholar]
- Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. Journal of Electromyography and Kinesiology. 2000;10(5):361–74. doi: 10.1016/s1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- Hof AL. EMG and muscle force: an introduction. Human Movement Science. 1984;3(1–2):119–53. [Google Scholar]
- Hortobágyi T, Westerkamp L, Beam S, Moody J, Garry J, Holbert D, DeVita P. Altered hamstring-quadriceps muscle balance in patients with knee osteoarthritis. Clinical Biomechanics. 2005;20(1):97–104. doi: 10.1016/j.clinbiomech.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Kellis E. Quantification of quadriceps and hamstring antagonist activity. Sports Medicine. 1998;25(1):37–62. doi: 10.2165/00007256-199825010-00004. [DOI] [PubMed] [Google Scholar]
- Kellis E, Baltzopoulos V. Agonist and antagonist EMG-angle relationship during isokinetic eccentric and concentric exercise. Isokinetics and Exercise Science. 1996;6:79–87. [Google Scholar]
- Kellis E, Katis A. Reliability of EMG power-spectrum and amplitude of the semitendinosus and biceps femoris muscles during ramp isometric contractions. Journal of Electromyography and Kinesiology. 2008;18(3):351–8. doi: 10.1016/j.jelekin.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Kellis E, Zafeiridis A, Amiridis IG. Muscle coactivation before and after the impact phase of running following isokinetic fatigue. Journal of Athletic Training. 2011;46(1):11–19. doi: 10.4085/1062-6050-46.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson L, Soderberg G, Ballantyne B, Clarke WR. A study of various normalisation procedures for within day electromyographic data. Journal of Electromyography and Kinesiology. 1994;4(1):47–59. doi: 10.1016/1050-6411(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Kubo K, Tsunoda N, Kanehisa H, Fukunaga T. Activation of agonist and antagonist muscles at different joint angles during maximal isometric efforts. European Journal of Applied Physiology. 2004;91(2–3):349–52. doi: 10.1007/s00421-003-1025-x. [DOI] [PubMed] [Google Scholar]
- Levine MG, Kabat H. Cocontraction and reciprocal innervations in voluntary movement in man. Science. 1952;116(3005):115–8. doi: 10.1126/science.116.3005.115. [DOI] [PubMed] [Google Scholar]
- Macaluso A, De Vito G. Muscle strength, power and adaptations to resistance training in older people. European Journal of Applied Physiology. 2004;91(4):450–72. doi: 10.1007/s00421-003-0991-3. [DOI] [PubMed] [Google Scholar]
- McKenzie LF, Petushek EJ, Feldmann CR, Hsu BE, Garceau LR, Lutsch BN, Ebben WP. Reliability of surface electromyography during maximal voluntary isometric contractions, jump landings and cutting. Journal of Strength and Conditioning Research. 2010;24(4):1131–7. doi: 10.1519/JSC.0b013e3181cc2353. [DOI] [PubMed] [Google Scholar]
- Merletti R, Parker PJ. Electromyography: Physiology, Engineering, and Non-Invasive Applications. Hoboken: John Wiley & Sons; 2004. [Google Scholar]
- Osternig LR, Hamill J, Corcos DM, Lander J. Electromyographic patterns accompanying isokinetic exercise under varying speed and sequencing conditions. American Journal of Physical Medicine and Rehabilitation. 1984;63(6):289–97. [PubMed] [Google Scholar]
- Seyedali M, Czerniecki JM, Morgenroth DC, Hahn ME. Co-contraction patterns of trans-tibial amputee ankle and knee musculature during gait. Journal of Neuroengineering and Rehabilitation. 2012;11(5):601–13. doi: 10.1186/1743-0003-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleivert GG, Wenger HA. Reliability of measuring isometric and isokinetic peak torque, rate of torque development, integrated electromyography and tibial nerve conduction velocity. Archives of Physical Medicine and Rehabilitation. 1994;75(12):1315–21. [PubMed] [Google Scholar]
- Solomonow M, Baratta R, Zhou BH, Shoji H, Bose W, Beck C, D’Ambrosia R. The synergistic action of the anterior cruciate ligament and thigh muscles in maintaining joint stability. The American Journal of Sports Medicine. 1987;15(3):207–13. doi: 10.1177/036354658701500302. [DOI] [PubMed] [Google Scholar]
- Van Dijk JP, Lowery MM, Lapatki BG, Stegeman DF. Evidence of potential averaging over the finite surface of a bioelectric surface electrode. Annals of Biomedical Engineering. 2009;37(6):1141–51. doi: 10.1007/s10439-009-9680-7. [DOI] [PubMed] [Google Scholar]
- Viitasalo JH, Komi PV. Signal characteristics of EMG with special reference to reproducibility of measurements. Acta Physiolica Scandinavica. 1975;93(4):531–9. doi: 10.1111/j.1748-1716.1975.tb05845.x. [DOI] [PubMed] [Google Scholar]
- Winter DA, Fuglevand AJ, Archer SE. Crosstalk in surface electromyography: Theoretical and practical estimates. Journal of Electromyography and Kinesiology. 1994;4(1):15–26. doi: 10.1016/1050-6411(94)90023-X. [DOI] [PubMed] [Google Scholar]
- Yang J, Winter D. Electromyographic amplitude normalisation methods: improve their sensitivity as diagnostic tools in gait analysis. Archives of Physical Medicine and Rehabilitation. 1984;65(9):517–21. [PubMed] [Google Scholar]
- Zech A, Witte K, Pfeifer K. Reliability and performance-dependent variations of muscle function variables during isometric knee extension. Journal of Electromyography and Kinesiology. 2008;18(2):262–69. doi: 10.1016/j.jelekin.2006.08.013. [DOI] [PubMed] [Google Scholar]