Abstract
Reduced expression of somatostatin (SST) is reported across chronic brain conditions including major depression and normal aging. SST is a signaling neuropeptide and marker of gamma-amino butyric acid (GABA) neurons, which specifically inhibit pyramidal neuron dendrites. Studies in auditory cortex suggest that chronic reduction in dendritic inhibition induces compensatory homeostatic adaptations that oppose the effects of acute inhibition. Whether such mechanisms occur in frontal cortex (FC) and affect behavioral outcome is not known. Here, we used two complementary viral vector strategies to examine the effects of acute vs chronic inhibition of SST-positive neurons on behavioral emotionality in adult mice. SST-IRES-Cre mice were injected in FC (prelimbic/precingulate) with CRE-dependent adeno-associated viral (AAV) vector encoding the engineered Gi/o-coupled human muscarinic M4 designer receptor exclusively activated by a designer drug (DREADD-hM4Di) or a control reporter (AAV-DIO-mCherry) for acute or chronic cellular inhibition. A separate cohort was injected with CRE-dependent AAV vectors expressing diphtheria toxin (DTA) to selectively ablate FC SST neurons. Mice were assessed for anxiety- and depressive-like behaviors (defined as emotionality). Results indicate that acute inhibition of FC SST neurons increased behavioral emotionality, whereas chronic inhibition decreased behavioral emotionality. Furthermore, ablation of FC SST neurons also decreased behavioral emotionality under baseline condition and after chronic stress. Together, our results reveal opposite effects of acute and chronic inhibition of FC SST neurons on behavioral emotionality and suggest the recruitment of homeostatic plasticity mechanisms that have implications for understanding the neurobiology of chronic brain conditions affecting dendritic-targeting inhibitory neurons.
INTRODUCTION
The function of the cerebral cortex relies on complex local circuits of interconnected excitatory glutamate pyramidal and inhibitory gamma-amino butyric acid (GABA) neurons. Although GABA neurons represent a minority of cortical neurons (10–20% in rodents) (Rudy et al, 2011), their dense axonal arborization across the dendritic tree, soma, and axon initial segment allows them to shape and regulate the entire network dynamic (Fino et al, 2013). Cortical GABA neurons have diverse and heterogeneous molecular, structural, and electrophysiological features, and changes in biological components of the excitation/inhibition balance are hypothesized to contribute to normal aging (Sibille, 2013) and to the development of several diseases, including epilepsy and neuropsychiatric disorders such as schizophrenia and major depressive disorder (MDD) (Croarkin et al, 2011; Lewis et al, 2005; Lin and Sibille, 2013; Luscher et al, 2011).
Somatostatin (SST)-positive GABA neurons mostly target the dendritic compartment of pyramidal cells and are recruited in a feedforward manner by activated pyramidal neurons for which they provide feedback inhibition (Fino et al, 2013). SST neurons (also called SOM neurons) are thus specialized in customized and targeted local regulation of incoming excitatory signals, and are critical in maintaining the integrity of information input (Fino et al, 2013). Recent findings (albeit in rodent visual cortex) suggest that SST neurons also provide a significant inhibition to most other GABA neuron subtypes (Pfeffer et al, 2013), and may thus regulate the overall inhibitory tone within local circuits in addition to regulating dendritic inhibition. Studies in auditory cortex of mice with conditional adult deletion of dendritic-targeting neurons show increased neuronal firing rate and tone-evoked responses (Seybold et al, 2012), consistent with acute pharmacological inhibition, but smaller frequency-intensity receptive fields with narrower bandwidths, which was associated with reduced corticocortical excitatory drive. These results provide evidence for homeostatic plasticity mechanisms limiting the impact of chronic loss of dendritic inhibition through the downregulation of corticocortical drive, hence suggesting a concerted and adaptive neural network response. These results are relevant when designing experiments aimed at investigating the contribution of SST-positive neurons to adult brain conditions, as reports of reduced SST expression often implicate several brain regions.
In MDD, converging evidence suggests a contribution of decreased GABA to the altered excitation/inhibition balance, as observed by proton magnetic resonance spectroscopy studies or by transcranial magnetic stimulation (Bajbouj et al, 2006; Hasler et al, 2007; Levinson et al, 2010; Sanacora et al, 1999; Sanacora et al, 2002). Studies of gene expression further support altered GABA-mediated inhibition in MDD (Luscher et al, 2011; Sequeira and Turecki, 2006; Tripp et al, 2011). Reduced SST expression was reported in the anterior cingulate cortex (Tripp et al, 2011; Tripp et al, 2012), dorsolateral prefrontal cortex (Sibille et al, 2011) and amygdala (Guilloux et al, 2012) of MDD subjects. These results are consistent with prior findings of decreased density in calbindin-positive GABA neurons in the dorsolateral prefrontal and orbitofrontal cortex (Rajkowska, 2000; Rajkowska et al, 2007), since SST is mostly colocalized with calbindin. These findings are supported by causal studies in mice, where mild reduction in GABA signaling is sufficient to induce anxiety- and depressive-like behaviors (Shen et al, 2010), together supporting a GABA deficit hypothesis of MDD (Luscher et al, 2011). However, reduced SST is also observed during normal aging (Erraji-Benchekroun et al, 2005), whereas depression-related symptoms are frequently observed, but not obligatory hallmarks of aging (Fiske et al, 2009). This suggests that distinct biological contexts and homeostatic mechanisms may moderate putative phenotypes associated with low SST.
In this study, we investigated whether acute or chronic reduction in SST-positive cell activity restricted to the frontal cortex (FC; including precingulate and prelimbic cortices) affects anxiety- and depression-like behaviors in adult mice. We applied two viral vector strategies to acutely or chronically inhibit the function of SST-positive GABA neurons in FC. First, we used the DREADD method (Designer Receptors Exclusively Activated by Designer Drugs) for acute and chronic pharmacogenetic neuronal inhibition (Alexander et al, 2009; Armbruster et al, 2007; Wess et al, 2013a). These studies were complemented by chemical ablation of SST neurons using an AAV vector designed to express diphtheria toxin A fragment (DTA) in a CRE-dependent manner in FC SST neurons. Mice with reduced SST-positive GABA neuron function restricted to FC were then submitted to a panel of behavioral tests for anxiety- and depressive-like behaviors, collectively designated as behavioral emotionality. Based on the above-mentioned studies in auditory cortex, we predicted that acute inhibition of FC SST-positive GABA neurons would increase behavioral emotionality and that homeostatic mechanisms may reduce this effect under chronic conditions of reduced dendritic inhibition.
MATERIALS AND METHODS
Mice
SST-ires-cre knock-in homozygous mice line (Ssttm2.1(cre)Zjh/J, Jackson Laboratories), in which CRE recombinase is driven by the endogenous Sst promoter (Taniguchi et al, 2011), was crossed with Ai6 Rosa26-loxP-STOP-loxP-ZsGreen reporter mice (B6.Cg-Gt(ROSA)26Sortm6(CAG-ZsGreen1)Hze/J), resulting in enhanced green fluorescent protein (ZsGreen) expression following Cre-mediated recombination (Madisen et al, 2010) selectively in SST neurons (Figure 1), and in a pattern consistent with endogenous gene expression (http://mouse.brain-map.org/). For the DTA experiment, SST-ires-cre knock-in homozygous mice line was crossed with ROSA26-loxP-STOP-loxP-LacZ reporter mice (B6.129S4-Gt(ROSA)26Sortm1Sor/J). All experiments were performed on adult mice (16–20 weeks of age at surgery). Male and female mice were used. There were no effects of sex on any measures, so groups were combined. Mice were maintained under standard conditions (12/12 h light/dark cycle, 22±1 °C, food and water ad libitum; 4 mice per cage) in accordance with the University of Pittsburgh Institutional Animal Care and Use Committee.
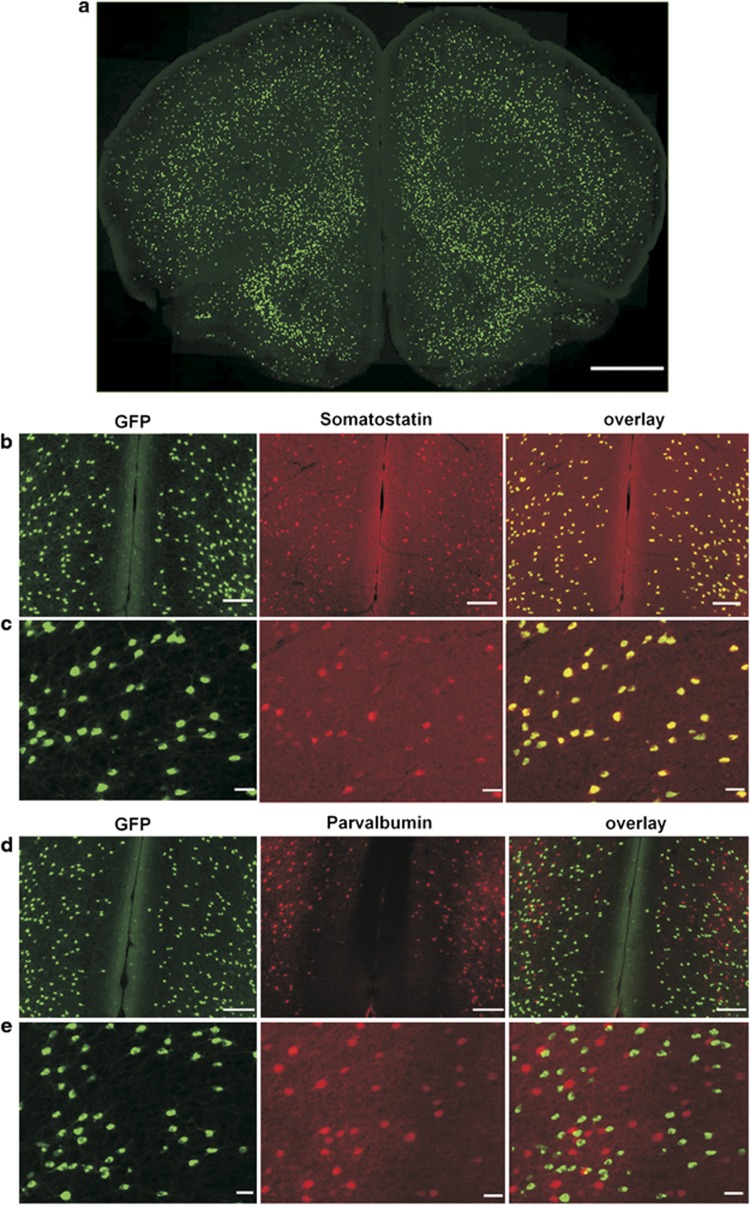
Figure 1.
SST-cre:Ai6 mice allows conditional manipulation of somatostatin (SST) expressing neurons. (a) GFP expression in a frontal cortex (FC) coronal section from an adult SST-ires-cre mice crossed with a Zsgreen cre-dependent reporter mice. Scale bar: 1 mm. (b, c) Immunostaining of GFP (green) and SST (red) shows colocalization in cortical neurons (overlay, yellow). (d, e) Immunostaining of GFP (green) and parvalbumin (red) in GFP-expressing neurons (overlay) reveals non-overlapping expression. Scale bars: 1 mm in (a), 100 μm in (b, d) and 20 μm in (c, e). A full color version of this figure is available at the Neuropsychopharmacology journal online.
AAV Vectors
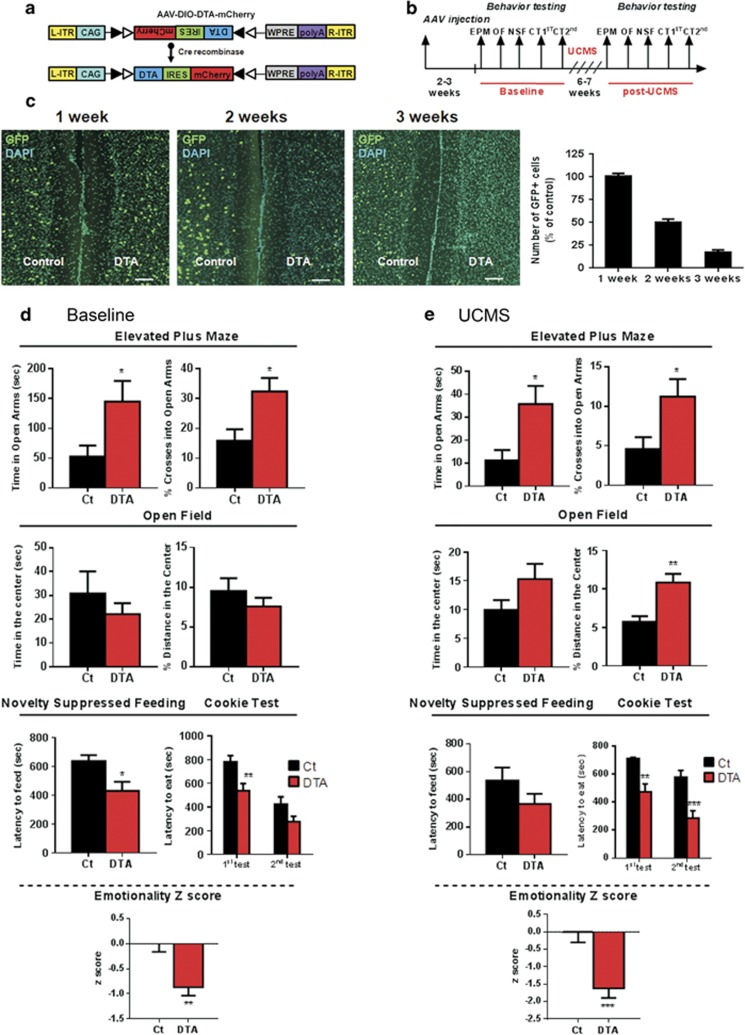
AAV DREADD vectors (AAV5-DIO-hM4Di-mCherry and AAV5-DIO-mCherry; Figure 2a) were obtained from the University of North Carolina Vector Core Facilities (Chapel Hill, NC). The AAV9-DTA-ires-mCherry was produced by Virovek (Hayward, CA) and generated by inserting the DTA-coding sequence into the pAAV-ires-hrmCherry vector (Figure 5a; Supplements).
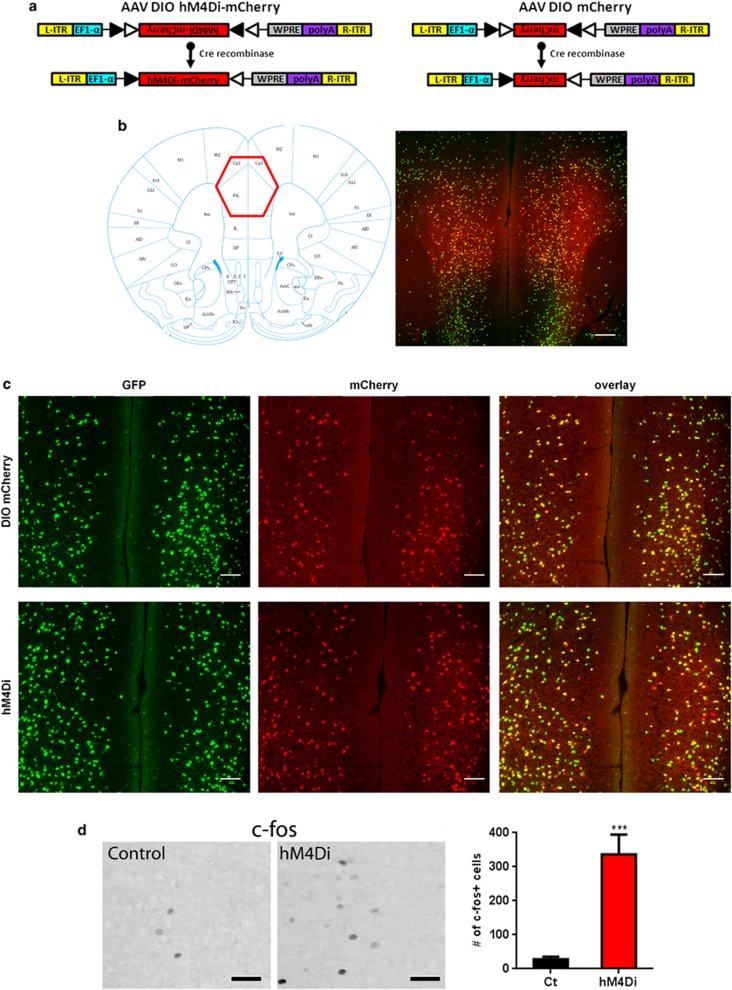
Figure 2.
Cortical SST cell targeting by DREADD-hm4Di. Panel (a) represents the design of hM4Di-mCherry AAV (left) and mCherry AAV (right) vectors employing the DIO strategy. Two pairs of heterotypic, antiparallel loxP recombination sites achieve cre-mediated transgenes inversion and expression under the control of EF1α promoter. L-ITR: left-inverted terminal repeat, EF1α: Human elongation factor-1 alpha, WPRE: woodchuck hepatitis virus post-transcriptional regulatory element, R-ITR: right-inverted terminal repeat. (b) Schematic coronal section illustrating the injection site (left) of the imaged area in the FC. mCherry fluorescence was detected in the cingulate and prelimbic areas following bilateral injection into SST-cre:Ai6 mice (right). Scale bar: 500 μm. (c) mCherry (top) and hM4Di receptors (bottom) were selectively/exclusively expressed in cortical SST neurons as illustrated by GFP (green) and mCherry (red) co-expression (overlay, yellow). Scale bar: 100 μm. (d) Representative c-fos immunoreactivity from SST:cre;Ai6 mice with mCherry (control) or hm4Di AAV vectors, 90 min following CNO injection (5 mg/kg, i.p.). Scale bars: 40 μm. Activation of hm4Di receptors by CNO increased the number of c-fos+ cells (***p<0.0001, n=6/8 mice per group). Data are mean±SEM. A full color version of this figure is available at the Neuropsychopharmacology Journal online.
Stereotaxic Viral Vector Injection
SST-ires-Cre:Ai61 mice were injected bilaterally with 800 nl of AAV5-DIO-hM4Di-mCherry virus (‘hm4Di') or with AAV5-DIO-mCherry (control virus) (∼1012 genome copies/ml) into the medial PFC (cingulate and prelimbic cortices) (Paxinos and Franklin, 2001). Coordinates were antero-posterior +1.94 mm; medio-lateral ±0.35 mm; for the dorso-ventral coordinates, two ventral coordinates were used to deliver 400 nl at −2.1 mm and 400 nl at −1.7 mm. Behavioral studies were conducted 2–3 weeks post injection. The range of post-operative time reflects the fact that surgery occurred over a few days. The same procedure was applied for the AAV-DTA experiments, in which animals received 800 nl of AAV9-DTA-ires-mCherry (‘DTA') or AAV9-mCherry (control virus), with a titer of ∼1013 genome copies per ml.
Pharmacogenetic Inhibition of SST Neurons
Mice injected with AAV-DIO-hM4Di-mCherry or AV-DIO-mCherry were intraperitoneally injected with clozapine-N-oxide (CNO) dissolved in 0.9% saline (Guettier et al, 2009; Wess et al, 2013b). Control mice were similarly injected with CNO. For acute experiments, mice received 5 mg/kg of CNO 30 min before behavioral testing, based on studies reporting high in vivo efficacy at 1–10 mg/kg doses following viral delivery (Atasoy et al, 2012; Ferguson et al, 2011; Sasaki et al, 2011). For chronic manipulation, mice received 0.5 mg/kg of CNO twice a day based on Kozorovitskiy et al (2012) and Krashes et al (2011).
Immunohistochemistry
Injection sites were verified for all mice. Because intrinsic fluorescence of mCherry was low in hM4Di mice, immunostaining against mCherry was performed to visualize the receptor localization. Paraformaldehyde-fixed 30 μm sections were incubated with a rabbit anti-red fluorescent protein (1 : 1000, Antibodies Online), followed by visualization with donkey anti-rabbit Cy3 conjugate (1 : 250, Millipore). SST was visualized using a rat anti-somatostatin (1 : 250, Millipore) and Parvalbumin+ cells with a rabbit anti-Parvalbumin, (1 : 1000, Millipore), followed by donkey anti-rat Cy3 conjugate and donkey-anti rabbit Cy3 conjugate (1 : 250, Millipore), respectively. Images were acquired using an epifluorescent microscope (Olympus, Nikon) and processed with Metamorph (Molecular Devices, Sunnyvale, CA).
c-Fos Staining
Stimulation of Gi/o-coupled hM4D receptors activates potassium channels, resulting in hyperpolarization and transient neuronal silencing following application of CNO (Armbruster et al, 2007; Ferguson et al, 2011; Rogan and Roth, 2011), hence decreasing inhibition on nearby neurons, as measured with expression of the immediate early gene c-fos (Rogan and Roth, 2011). Control and hM4Di mice were injected with CNO (5 mg/kg, i.p.) and perfused 90 min later with saline and 4% paraformaldehyde. Free-floating coronal sections were immunostained using a rabbit anti-c-Fos antibody (1 : 500, overnight, Calbiochem), followed by secondary goat anti-rabbit IgG (1 : 200, Vector Laboratories), avidin-biotin-horseradish peroxidase kit (Vector Laboratories), and visualized with diaminobenzidine solution (DAB peroxidase substrate kit; Vector Laboratories). c-Fos-positive cells were counted in cingulate and prelimbic areas using a × 20 objective on a 1 : 5 series (4 sections/animal) through the entire infected PFC (∼800 μm) and multiplied by 5 to estimate the total cell number.
Behavioral Testing
Elevated plus maze
Behavior was measured using a cross maze with two open and two closed 30 × 5 cm arms. Time spent in and percent entries into the open arms were recorded for 10 min to measure anxiety-like behavior. The total number of entries into any arm was used as an index of locomotion.
Open field
The open field (OF) test was performed in a 50.8 × 50.8 cm arena, and the center of the OF was defined as the centermost 25.4 × 25.4 cm of the arena. ANY-Maze software (Stoelting) was used to track behavior using a ceiling-mounted video camera. Time spent and percent distance in the center of the arena were recorded for 10 min, as this block of time captures the initial anxiety-like component of the test. The total distance traveled over 30 min was recorded as an index of locomotor activity.
Novelty suppressed feeding
Mice were food deprived for 16 h (with ad libitum water) before NSF exposure. Testing was performed in a brightly lit 51 × 51 cm arena covered in bedding. Latency to eat a regular food pellet placed in the aversive center of the arena was recorded during a 12-min session, as an index of anxiety-/depressive-like behavior (collectively referred to as ‘emotionality'). Food consumption in the home cage during 8 min following NSF testing and percent weight lost during food deprivation were measured as controls for feeding drive.
Cookie test
The test assesses the motivation for a palatable stimulus (chocolate cookie) (Isingrini et al, 2011). This test used three aligned chambers of similar dimensions (20 × 20 × 20 cm), but different wall colors (white, gray, and black). Mice were familiarized with the chocolate cookie in their home cage (2 g±1; Keebler Fudge Stripe) for 5 days. For testing, a small amount of cookie was placed in the center of the black compartment and the mouse was placed in the opposite white compartment. The test was performed as described in Isingrini et al (2011) with the modification that the latency to begin eating the cookie was recorded instead of number of bites. Each session lasted 12 min and two sessions of testing were performed with an inter-test interval of 2 days. As mice acclimate to the environment, the second testing is often characterized by a shorter latency to begin eating, which is interpreted as having a lower anxiogenic effect and greater component of consumption of a palatable food or anhedonia-related behavior.
Sucrose preference test
Mice were habituated to sucrose by giving them free access to water and 1% sucrose solution for 48 h. The location of the sucrose bottles was balanced across each animal and alternated after 24 h. Following training, mice were single-housed in a new cage for 16 h (during the active phase) and given free access to both water and sucrose solution. Sucrose and water consumption were measured, and sucrose preference was calculated as the ratio of sucrose consumed over total fluid consumption.
Emotionality z-score
To assess the consistency of behavioral performance over time and related assays, emotionality-related measures were Z-normalized across related tests as described (Guilloux et al, 2011). These scores have lower test-specific sensitivity and are interpreted as overall behavioral emotionality. Briefly, Z-scores are standardized to the mean and standard deviation of a comparison group. Z-scores indicate how many standard deviations an observation is above or below the mean of the comparison group, with directions of changes determined by the respective emotionality measures of the tests. Test-specific Z-scores are first averaged within then across tests.
Unpredictable chronic mild stress
Unpredictable chronic mild stress (UCMS) replicates the known role of chronic socio-environmental stress in eliciting depressive episodes in humans, models several symptom dimensions, and respects the timeframe of onset and efficacy of antidepressant treatment (Surget et al, 2009). Briefly, the UCMS protocol consisted of a 6-week period during which group-housed mice were exposed to a randomized schedule of environmental disturbances ∼1–3 times per day, 7 days a week (Edgar et al, 2011; Surget et al, 2009). Disturbances included forced bath (∼2 cm of water), aversive smell (bobcat urine), light cycle reversal or disruption, social stress (rotate mice into previously occupied cages), tilted cage, mild restraint, bedding change, wet bedding, and no bedding. Weekly assessment of weight and fur was performed to track progression of the UCMS syndrome (Surget et al, 2009).
Data Analysis
Statistical analyses were performed using two-way ANOVA with Bonferroni post hoc tests (for CT test; adjusted p-values are reported), or Student's t-test as appropriate. All data are expressed as mean±SEM and statistical significance was set at p<0.05.
RESULTS
Selective Targeting of SST Neurons using the DREADD Approach
The Cre dependency and cellular specificity of the SstCre line (Taniguchi et al, 2011) was verified by immunostaining (Figure 1a). GFP expression was restricted to SST-positive neurons as demonstrated by the colocalization with SST immunostaining (Figure 1b and c) and lack of overlap with parvalbumin immunostaining, a non-overlapping subtype of GABA neurons (Figure 1d and e). To inhibit SST neuronal activity, we used AAV vectors containing DREADD-hM4Di that is activated by a high affinity, and otherwise pharmacologically inert, ligand CNO (Armbruster et al, 2007; Wess et al, 2013a). The hM4Di coding sequence was packaged into a cre-recombinase-dependent AAV vector that enables the expression of the red fluorescent protein mCherry (AAV-DIO-hM4Di-mCherry) which was used as a control vector (Figure 2a). Following stereotaxic injection of either AAV-DIO-hM4Di-mCherry or AAV-DIO-mCherry in the FC (cingulate and prelimbic areas, Figure 2b), red fluorescent mCherry signaling was colocalized with GFP-positive cells (Figure 2c). Injection of these constructs into wild-type mice revealed no detectable mCherry expression (not shown).
hM4Di activation by CNO results in hyperpolarization and transient neuronal silencing (Armbruster et al, 2007; Ferguson et al, 2011; Rogan and Roth, 2011). To validate the DREADD technique in vivo in our experimental condition, we determined whether inhibiting SST cell activity would mediate disinhibition and elicit neuronal activity in nearby cells, using c-fos expression as an indicator of neuronal activity. As predicted, acute administration of CNO triggered c-fos expression in large numbers of cells within the injected areas (t=6.54, df=13, p<0.0001) (Figure 2d).
Acute Pharmacogenetic Inhibition of Cortical SST Neurons Increases Anxiety-Like Behavior and Overall Emotionality
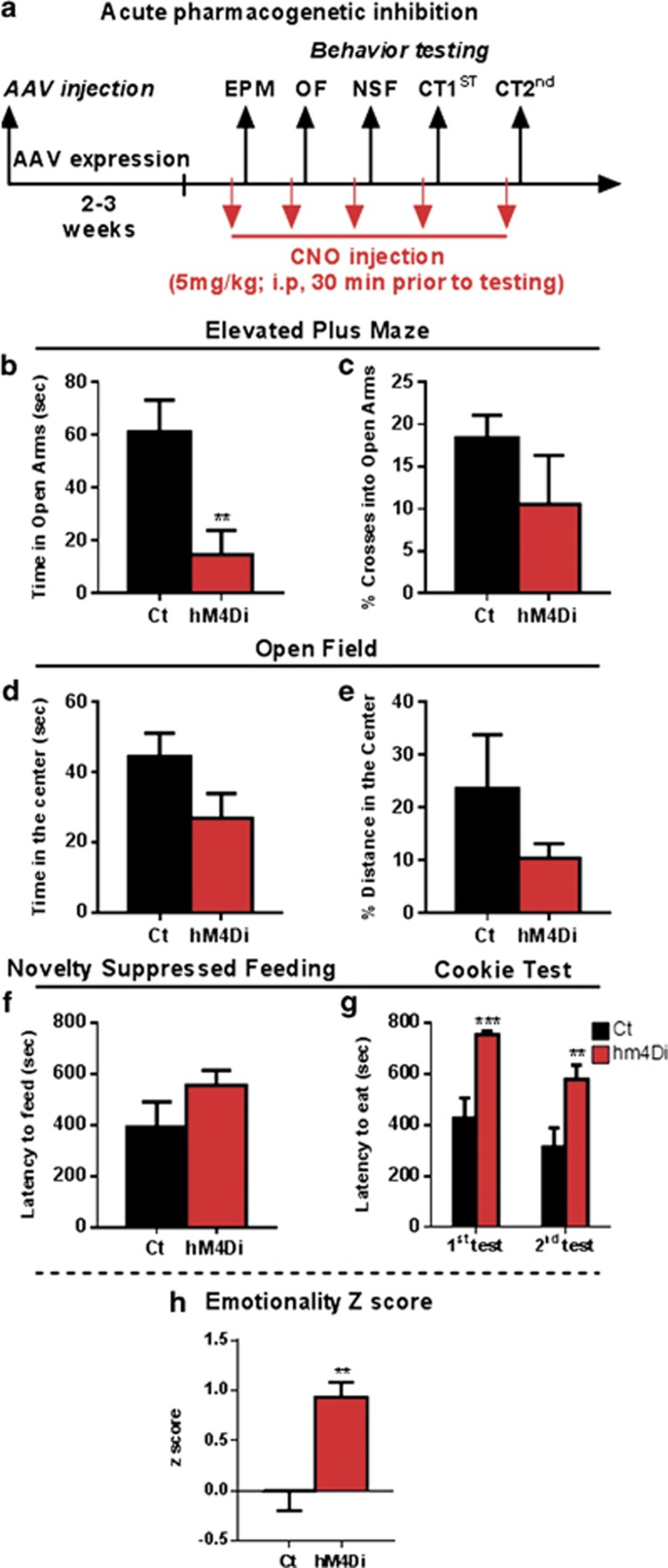
The effect of acute CNO injection on anxiety- and depressive-like behaviors was analyzed using the elevated plus maze (EPM), OF, novelty suppressed feeding (NSF), and cookie test (CT) (Figure 3a). Compared with control mice, hM4Di mice showed significant reduced time spent in the aversive open arms of the EPM (t=3.100, df=17, p=0.006; Figure 3b) and a non-significant decrease in percent crosses into the open arms (t=1.191, df=17, p=0.25; Figure 3c). In the OF, a trend to reduce time spent in the aversive center was observed (t=1.794, df=17, p=0.09; Figure 3d) and no effect on the percent distance traveled in the center (t=1.300, df=17, p=0.21; Figure 3e). While hm4Di mice showed no difference in the latency to feed in the NSF test (t=1.445, df=17, p=0.16; Figure 3f), a significant increase in the latency to begin eating was observed in the two sessions of CT (F(1,17)=16.6, p=0.0008 for main effect of Di/CNO; F(1,17)=14.99, p=0.0017 for main effect of session, and F(1,17)=0.79, p=0.3846 for their interaction; Figure 3g). To investigate the consistency of behavioral performance across tests, we performed z-scoring of related behavioral measures using the mean value of the control group as baseline (Guilloux et al, 2011). hm4Di mice showed significant elevated emotionality z-scores (t=3.806, df=17, p=0.001; Figure 3h), confirming that acute inhibition of FC SST neurons is sufficient to induce elevated anxiety-like and overall behavioral emotionality.
Figure 3.
Acute pharmacogenetic inhibition of SST neurons increases anxiety-like behavior and overall emotionality. (a) Schematic of the experimental design used to study the acute effect of SST cells inhibition on behavior. All mice were CNO treated (5 mg/kg, i.p.) 30 min before behavioral testing. Behavioral results associated with acute pharmacogenetic inhibition of SST cells on elevated plus maze (time spent in open arms in b, percent (%) of crosses into open arms in c), open field (time spent in the center in d, % of distance traveled in the center in e), latency to eat in the novelty-suppressed feeding (f) and cookie test (g). (h) Resulting integrated emotionality z-score (**p<0.01, ***p<0.0001, n=9/10 mice per group; data are mean±SEM). A full color version of this figure is available at the Neuropsychopharmacology journal online.
Chronic Pharmacogenetic Inhibition of Cortical SST Neurons Reduces Anxiety-Like Behavior and Overall Emotionality
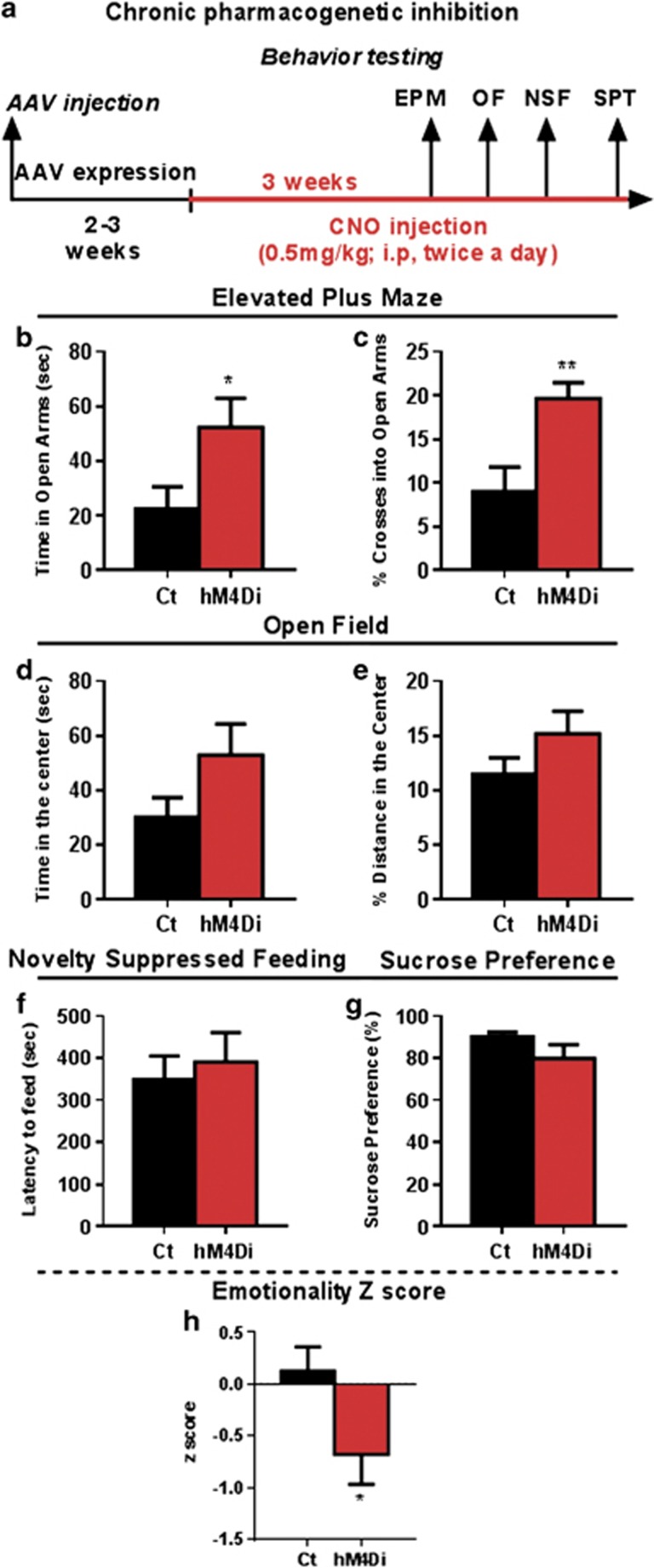
As chronic changes in excitation/inhibition balance can induce circuit level homeostatic changes (Seybold et al, 2012), we next assessed the impact of chronic inhibition of SST neurons on behavioral emotionality. Mice with bilateral AAV-hM4Di injection were treated with CNO daily for 3 weeks (Figure 4a). Upon chronic CNO delivery, hm4Di mice showed significant increases in time spent (t=2.207, df=14, p<0.05; Figure 4b) and percent of crosses in the open arms of the EPM (t=3.133, df=14, p<0.01; Figure 4c). No significant effects were observed in the OF (time spent in the center: t=1.679, d=14, p=0.11; or in percent distance traveled in the center (t=1.430, df=14, p=0.17; Figure 4d and e). No differences were observed in the latency to feed in the NSF (t=0.461, df=14, p=0.65; Figure 4f) and in sucrose preference (t=1.472, df=14, p=0.16; Figure 4g). Assessing the consistency of behavioral performance across tests through behavioral z-scoring, mice with chronic CNO injection displayed a significantly lower score (t=2.156, df=14, p<0.05; Figure 4h), indicating that chronic inhibition of SST neurons resulted in reduced behavioral emotionality. There was no evidence for toxic effects of chronic CNO application, as evidenced by the absence of overt cellular changes at the sites of injection (cellular damage, necrosis), and of physiological (coat or weight changes) or locomotor activity effects (ie, distance traveled in the OF; Supplementary Figure S1).
Figure 4.
Chronic pharmacogenetic inhibition of SST neurons reduces anxiety-like behavior and overall emotionality. (a) Schematic of the experimental design used to study the chronic effect of SST cells inhibition on behavior. Mice received a dose of CNO (0.5 mg/kg, i.p., twice a day, 8 h apart). Behavioral results associated with chronic pharmacogenetic inhibition of SST cells on elevated plus maze (time spent in open arms in b and % of crosses into open arms in c), open field (time spent in the center in d and % of distance traveled in the center in e), latency to eat in the novelty-suppressed feeding (f) and cookie test (g). (h) Resulting integrated emotionality z score (*p<0.05, **p<0.01, n=8 mice per group; data are mean±SEM). A full color version of this figure is available at the Neuropsychopharmacology journal online.
Selective Ablation of Cortical SST Neurons Reduces Anxiety-Like Behavior and Overall Emotionality under Baseline and Chronic Stress Conditions
In view of the opposing behavioral effects of acute and chronic SST silencing, we sought to independently verify these results using a complementary approach aimed at deleting FC SST neurons. We used conditional expression of a DTA to selectively ablate neurons (Collier, 2001; Palmiter et al, 1987). We developed a cre-dependent AAV vector carrying a double-floxed inverted open reading frame containing DTA and the mCherry red reporter gene (AAV-DIO-DTA-ires-mCherry, Figure 5a). To validate the approach, SST-ires-Cre;Ai6 mice received unilateral DTA injection into the FC and the number of SST-expressing GFP cells was compared with the vehicle-injected contralateral side (Figure 5c). While no effect was observed 1 week after DTA injection, the numbers of GFP-positive SST neurons decreased by 50% at 2 weeks, and ∼90% at 3 weeks post injection, consistent with a DTA-mediated ablation of SST-positive neurons.
Figure 5.
Selective ablation of cortical SST neurons reduces anxiety-like behavior and overall emotionality under baseline and chronic stress conditions. (a) Design of DTA-mCherry AAV employing the DIO strategy, which uses two pairs of heterotypic, antiparallel loxP recombination sites to achieve cre-mediated transgenes inversion and expression under the control of CAG promoter. L-ITR: left-inverted terminal repeat, CAG: CMV early enhancer/chicken beta actin, WPRE: woodchuck hepatitis virus posttranscriptional regulatory element, R-ITR: right-inverted terminal repeat. (b) Schematic of the experimental design used to study the effect of SST cells suppression on behavior. Mice were tested twice: before (baseline) and after unpredictable chronic stress paradigm (UCMS). (c) Illustration of GFP (green) and DAPI (blue) immunostaining in the vehicle-control side and following 1, 2, and 3 weeks of DTA injection. The quantification of GFP cells 1, 2, and 3 weeks after DTA injection expressed as the percent of control (n=3 per condition) is shown to the right. Scale bar: 100 μm. Behavioral results associated with genetic ablation of SST cells under baseline condition (d) and after UCMS (e). Note that the longitudinal aspects of the study (pre- and post-stress in same cohorts), rather than cross-sectional, preclude direct comparison of pre- vs post-stress effects, as variations in baseline behavior 6–7 weeks apart frequently occur and could be misconstrued here as ‘stress effect' if presented together. Instead the contrasts of interest ‘DTA vs control treatments' are presented under baseline and stress conditions. (*p<0.05, **p<0.01, ***p<0.001 n=13/14 mice per group; data are mean±SEM). A full color version of this figure is available at the Neuropsychopharmacology journal online.
Mice were tested 3 weeks following bilateral AAV-DTA stereotactic injection (Figure 5b). Compared with control mice receiving AAV-DIO-mCherry, AAV-DTA-injected mice spent more time in the open arms of the EPM (t=2.068, df=25, p<0.05) and showed elevated percent crosses into the open arms (t=2.619, df=25, p<0.05) (Figure 5d). No differences were observed in the time and relative distance traveled in the center of the OF. AAV-DTA-injected mice displayed reduced latency to eat in the NSF (t=2.500, df=25, p<0.05) and in the CT (Main effects: DTA, F(1,25)=58.84, p<0.0001; Session, F(1,25)=7.764, p=0.0100; Interaction F(1,25)=1.407, p=0.24). Significant differences in emotionality z-score attested to the consistency of the performance across tests (t=3.501, df=25, p<0.01) (Figure 5d) and independently confirmed the previous results (Figure 4) following chronic loss of activity with the pharmacogenetic approach.
We next investigated the phenotype of AAV-DTA-injected mice under challenging conditions, namely their vulnerability to develop high behavioral emotionality following exposure to chronic stress. Following 6.5 weeks of UCMS (Figure 5e), AAV-DTA-injected mice exhibited significant increases in time (t=2.385, df=25, p<0.05) and percent crosses (t=2.244, df=25, p<0.05) into the EPM open arms, in the percent distance traveled in the OF center (t=3.421, df=25, p<0.01) and in latency to eat in the CT (Main effects: DTA, F(1,25)=13.33, p=0.0012; Session, F(1,25)=21.47, p<0.0001; Interaction, F(1,25)=0.3742). No significant differences were observed in the time spent in the OF center (t=1.532, df=25, p=0.13) and in the NSF (t=1.432, df=25, p=0.16). Emotionality z-scores were significantly lower for AAV-DTA-injected mice (t=3.890, df=25, p<0.01), highlighting the consistency of the behavioral responses, and confirming the lower emotionality phenotype observed under non-stressed baseline conditions (Figure 5d). There were no difference in the total distance traveled in the OF before and after UCMS (Supplementary Figure S1).
Together, these results confirmed findings after chronic pharmacogenetic inhibition of cortical SST neurons, and establish that chronic inhibition of SST neurons restricted to FC reduces behavioral emotionality and blocks its occurrence following chronic stress.
DISCUSSION
Using conditional and cell-specific targeted viral-mediated loss of function, we identified a causal role for frontal cortical SST cell activity in the expression of anxiety-like behavior and overall emotionality in adult mice. Specifically, we show that acute inhibition of SST cell activity in FC using CNO-/hM4Di-mediated cellular inhibition increased anxiety-like behavior and overall emotionality (Figure 3). Conversely, chronic inhibition of FC SST cell activity using pharmacogenetic inhibition (Figure 4) or genetic ablation (Figure 5) decreased anxiety-like behavior and emotionality. These latter results were also observed following exposure to chronic stress in mice with FC SST cell ablation. Together, this suggests a complex and time-dependent contribution of FC SST-positive GABA neurons to behavioral emotionality, with putative adaptations in local circuit and/or neural network functions during periods of chronic SST cell inhibition. The latter findings are consistent with prior studies in auditory cortex showing that acute blockade or deletion of dendritic-targeting neurons resulted in opposite changes in frequency-intensity receptive fields and bandwidths (Seybold et al, 2012). The present study now extends those acute/chronic observations to the FC and to anxiety-like and emotionality behavioral outcomes, consistent with plasticity of cortical brain circuitry in response to chronic reductions in inhibition.
Beyond its relevance to understand the acute role of SST cell function in cortical emotional processing, our findings first show that chronic conditions featuring changes in SST-cell inhibitory circuitry are not modeled by acute manipulations of cellular functions. Indeed, for major depression and other brain disorders, SST-related changes likely represent chronic conditions (Tripp et al, 2011, 2012) and are reported in the context of reduced expression of additional GABA neuron markers targeting pyramidal cell dendrites (ie, low Cortistatin and Neuropeptide Y) and across corticolimbic brain regions (dorsolateral prefrontal cortex, anterior cingulate, and amygdala) (Guilloux et al, 2012; Tripp et al, 2012). So it is reasonable to assume that the observed behavioral outcome of pathologies characterized by chronic molecular and cellular changes spanning multiple interconnected brain areas will involve complex local circuit and neural network adaptations that are not modeled by the current studies. Moreover, in a related study, optogenetic stimulation of FC neurons did not change anxiety- or depressive-like behaviors but reversed the social avoidance and a decrease in sucrose preference induced by chronic social defeat stress (Covington et al, 2010), indicating that the effects of SST-mediated disinhibition of local cell circuit differ from acute overall neuron stimulation, which altogether precludes any straightforward identification of mechanisms related to inhibition/excitation changes in chronic mood disorders.
It is not known whether adaptations in local cell circuits or in subcortical projections mediate the emergence of the opposite phenotype following chronic inhibition. The brain regions targeted in this study include prelimbic and precingulate cortices which project to and receive input from other brain regions, including areas implicated in the regulation of behavioral emotionality, notably the amygdala and the nucleus accumbens (Davidson, 2002; Vertes, 2004). Studies in auditory cortex showed that homeostatic control following reduced dendritic-targeting GABA neuron cell population was maintained through the downregulation of corticocortical excitatory projections, rather than through modifications of thalamic afferent, the other major source of cortical excitatory input. So we can speculate on a similar cortical origin of compensatory changes to maintain FC inhibition/excitation homeostasis, but follow-up studies mapping the recruitment levels of other regions and neural networks are needed to answer these questions.
There are several technical considerations to keep in mind. Using the DREADD approach, we showed opposite effects on behavior following acute or chronic inhibition of SST-positive interneurons. The two experiments used different doses of CNO, notably a lower dose for the chronic injection to avoid chronic desensitization. But the replication of the behavioral effects of the chronic inhibition by DTA-mediated ablation of FC SST neurons suggests that the opposite pharmacogenetic findings are not due to dose differences. We can also rule out a potential effect of retro-reduction of CNO to clozapine for several reasons. First, while CNO back metabolism to clozapine has been observed in humans and guinea pigs (Jann et al, 1994), it was not detected in rats and mice (Guettier et al, 2009; Jann et al, 1994). Second, CNO-treated control mice did not show any alteration in behaviors associated with clozapine exposure such as reduced locomotor activity (McOmish et al, 2012). Here, no difference was observed in the total distance traveled in the OF (Supplementary Figure S1). Third, we observed similar behavioral outcomes to chronic hm4Di activation from genetic ablation under baseline and after chronic stress. It is not known at this time whether the chronic effect is reversible, upon cessation of pharmacogenetic inhibition for instance. Finally, we used several behavioral tests with slight differences across experiments due to technical constraints (ie, SP in Figure 4 and CT in Figures 3 and 5). These tests assess the same construct of rodent emotionality, encompassing anxiety- and/or depressive-like behaviors. These dimensions are highly interconnected and subject to variability over time. For instance, the origins of the differences in behavioral responses between EPM and OF, or SP and CT, are not known. Both sets of tests assess similar constructs respectively, and differences in results could reflect test specificity or normal behavioral variance over time. So we opted for the additional integration of behavioral results (Guilloux et al, 2011) to obtain robust measures of overall behavioral emotionality, but we recognize that additional dimensions may be worth exploring in follow-up experiments.
In conclusion, we identified a causal role for frontal cortical SST cells in modulating behavioral emotionality. Moreover, the opposite behavioral effects of acute and chronic inhibition of FC SST neurons suggest the recruitment of homeostatic plasticity mechanisms that have implications for investigating the neurobiology of chronic brain conditions affecting dendritic-targeting inhibitory neurons.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
We thank Robert Skrinak for technical assistance, Beverly French for technical assistance and careful feedback on the manuscript, and Bryan Roth for conceptual and technical support for the DREADD experiments. Clozapine-N-oxide (CNO) was obtained from the NIH as part of the Rapid Access to Investigative Drug Program funded by the NINDS. This work was supported by National Institute of Mental Health MH084060 and MH077159 to ES.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajbouj M, Lisanby SH, Lang UE, Danker-Hopfe H, Heuser I, Neu P. Evidence for impaired cortical inhibition in patients with unipolar major depression. Biol Psychiatry. 2006;59:395–400. doi: 10.1016/j.biopsych.2005.07.036. [DOI] [PubMed] [Google Scholar]
- Brockschnieder D, Lappe-Siefke C, Goebbels S, Boesl MR, Nave KA, Riethmacher D. Cell depletion due to diphtheria toxin fragment A after Cre-mediated recombination. Mol Cell Biol. 2004;24:7636–7642. doi: 10.1128/MCB.24.17.7636-7642.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier RJ. Understanding the mode of action of diphtheria toxin: a perspective on progress during the 20th century. Toxicon. 2001;39:1793–1803. doi: 10.1016/s0041-0101(01)00165-9. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S, et al. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J Neurosci. 2010;30:16082–16090. doi: 10.1523/JNEUROSCI.1731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croarkin PE, Levinson AJ, Daskalakis ZJ. Evidence for GABAergic inhibitory deficits in major depressive disorder. Neurosci Biobehav Rev. 2011;35:818–825. doi: 10.1016/j.neubiorev.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- Edgar NM, Touma C, Palme R, Sibille E. Resilient emotionality and molecular compensation in mice lacking the oligodendrocyte-specific gene Cnp1. Transl Psychiatry. 2011;1:e42. doi: 10.1038/tp.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erraji-Benchekroun L, Underwood MD, Arango V, Galfalvy H, Pavlidis P, Smyrniotopoulos P, et al. Molecular aging in human prefrontal cortex is selective and continuous throughout adult life. Biol Psychiatry. 2005;57:549–558. doi: 10.1016/j.biopsych.2004.10.034. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, et al. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2011;14:22–24. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E, Packer AM, Yuste R. The logic of inhibitory connectivity in the neocortex. Neuroscientist. 2013;19:228–237. doi: 10.1177/1073858412456743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiske A, Wetherell JL, Gatz M. Depression in older adults. Annu Rev Clin Psychol. 2009;5:363–389. doi: 10.1146/annurev.clinpsy.032408.153621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guettier JM, Gautam D, Scarselli M, Ruiz de Azua I, Li JH, Rosemond E, et al. A chemical-genetic approach to study G protein regulation of beta cell function in vivo. Proc Natl Acad Sci USA. 2009;106:19197–19202. doi: 10.1073/pnas.0906593106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, et al. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry. 2012;17:1130–1142. doi: 10.1038/mp.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilloux JP, Seney M, Edgar N, Sibille E. Integrated behavioral z-scoring increases the sensitivity and reliability of behavioral phenotyping in mice: Relevance to emotionality and sex. J Neurosci Methods. 2011;197:21–31. doi: 10.1016/j.jneumeth.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- Isingrini E, Surget A, Belzung C, Freslon JL, Frisbee J, O'Donnell J, et al. Altered aortic vascular reactivity in the unpredictable chronic mild stress model of depression in mice: UCMS causes relaxation impairment to ACh. Physiol Behav. 2011;103:540–546. doi: 10.1016/j.physbeh.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Jann MW, Lam YW, Chang WH. Rapid formation of clozapine in guinea-pigs and man following clozapine-N-oxide administration. Arch Int Pharmacodyn Ther. 1994;328:243–250. [PubMed] [Google Scholar]
- Kozorovitskiy Y, Saunders A, Johnson CA, Lowell BB, Sabatini BL. Recurrent network activity drives striatal synaptogenesis. Nature. 2012;485:646–650. doi: 10.1038/nature11052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson AJ, Fitzgerald PB, Favalli G, Blumberger DM, Daigle M, Daskalakis ZJ. Evidence of cortical inhibitory deficits in major depressive disorder. Biol Psychiatry. 2010;67:458–464. doi: 10.1016/j.biopsych.2009.09.025. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lin LC, Sibille E. Reduced brain somatostatin in mood disorders: a common pathophysiological substrate and drug target. Front Pharmacol. 2013;4:110. doi: 10.3389/fphar.2013.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;16:383–406. doi: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McOmish CE, Lira A, Hanks JB, Gingrich JA. Clozapine-induced locomotor suppression is mediated by 5-HT(2A) receptors in the forebrain. Neuropsychopharmacol. 2012;37:2747–2755. doi: 10.1038/npp.2012.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD, Behringer RR, Quaife CJ, Maxwell F, Maxwell IH, Brinster RL. Cell lineage ablation in transgenic mice by cell-specific expression of a toxin gene. Cell. 1987;50:435–443. doi: 10.1016/0092-8674(87)90497-1. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ.2001The Mouse Brain in Stereotoxic Coordinates2nd ednAcademic Press: San Diego, CA [Google Scholar]
- Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci. 2013;16:1068–1076. doi: 10.1038/nn.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry. 2000;48:766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, O'Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ. GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacol. 2007;32:471–482. doi: 10.1038/sj.npp.1301234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan SC, Roth BL. Remote control of neuronal signaling. Pharmacol Rev. 2011;63:291–315. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol. 2011;71:45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, et al. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56:1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, Krystal JH. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry. 2002;159:663–665. doi: 10.1176/appi.ajp.159.4.663. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Suzuki M, Mieda M, Tsujino N, Roth B, Sakurai T. Pharmacogenetic modulation of orexin neurons alters sleep/wakefulness states in mice. PLoS ONE. 2011;6:e20360. doi: 10.1371/journal.pone.0020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira A, Turecki G. Genome wide gene expression studies in mood disorders. OMICS. 2006;10:444–454. doi: 10.1089/omi.2006.10.444. [DOI] [PubMed] [Google Scholar]
- Seybold BA, Stanco A, Cho KK, Potter GB, Kim C, Sohal VS, et al. Chronic reduction in inhibition reduces receptive field size in mouse auditory cortex. Proc Natl Acad Sci USA. 2012;109:13829–13834. doi: 10.1073/pnas.1205909109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Lal R, Luellen BA, Earnheart JC, Andrews AM, Luscher B. gamma-Aminobutyric acid-type A receptor deficits cause hypothalamic-pituitary-adrenal axis hyperactivity and antidepressant drug sensitivity reminiscent of melancholic forms of depression. Biol Psychiatry. 2010;68:512–520. doi: 10.1016/j.biopsych.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibille E. Molecular aging of the brain, neuroplasticity, and vulnerability to depression and other brain-related disorders. Dialogues Clin Neurosci. 2013;15:53–65. doi: 10.31887/DCNS.2013.15.1/esibille. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibille E, Morris HM, Kota RS, Lewis DA. GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int J Neuropsychopharmacol. 2011;14:721–734. doi: 10.1017/S1461145710001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surget A, Wang Y, Leman S, Ibarguen-Vargas Y, Edgar N, Griebel G, et al. Corticolimbic transcriptome changes are state-dependent and region-specific in a rodent model of depression and of antidepressant reversal. Neuropsychopharmacology. 2009;34:1363–1380. doi: 10.1038/npp.2008.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp A, Kota RS, Lewis DA, Sibille E. Reduced somatostatin in subgenual anterior cingulate cortex in major depression. Neurobiol Dis. 2011;42:116–124. doi: 10.1016/j.nbd.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp A, Oh H, Guilloux JP, Martinowich K, Lewis DA, Sibille E. Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am J Psychiatry. 2012;169:1194–1202. doi: 10.1176/appi.ajp.2012.12020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Wess J, Nakajima K, Jain S. Novel designer receptors to probe GPCR signaling and physiology. Trends Pharmacol Sci. 2013;34:385–392. doi: 10.1016/j.tips.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wess J, Nakajima K, Jain S. Novel designer receptors to probe GPCR signaling and physiology. Trends Pharmacol Sci. 2013;34:385–392. doi: 10.1016/j.tips.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.