Abstract
Objective
To determine if change in maternal angiogenic biomarkers between the first and second trimesters predicts pre-eclampsia in low-risk nulliparous women.
Design
A nested case–control study of change in maternal plasma soluble Flt-1 (sFlt-1), soluble endoglin (sEng) and placenta growth factor (PlGF). We studied 158 pregnancies complicated by pre-eclampsia and 468 normotensive nonproteinuric controls.
Setting
A multicentre study in 16 academic medical centres in the USA.
Population
Low-risk nulliparous women.
Methods
Luminex assays for PlGF, sFlt-1 and sEng performed on maternal EDTA plasma collected at 9–12, 15–18 and 23–26 weeks of gestation. Rate of change of analyte between first and either early or late second trimester was calculated with and without adjustment for baseline clinical characteristics.
Main outcome measures
Change in PlGF, sFlt-1 and sEng.
Results
Rates of change of PlGF, sEng and sFlt-1 between first and either early or late second trimesters were significantly different in women who developed pre-eclampsia, severe pre-eclampsia or early-onset pre-eclampsia compared with women who remained normotensive. Inclusion of clinical characteristics (race, body mass index and blood pressure at entry) increased sensitivity for detecting severe and particularly early-onset pre-eclampsia but not pre-eclampsia overall. Receiver operating characteristics curves for change from first to early second trimester in sEng, PlGF and sFlt-1 with clinical characteristics had areas under the curve of 0.88, 0.84 and 0.86, respectively, and for early-onset pre-eclampsia with sensitivities of 88% (95% CI 64–99%), 77% (95% CI 50–93%) and 77% (95%CI 50–93%) for 80% specificity, respectively. Similar results were seen in the change from first to late second trimester.
Conclusion
Change in angiogenic biomarkers between first and early second trimester combined with clinical characteristics has strong utility for predicting early-onset pre-eclampsia.
Keywords: Angiogenesis, endoglin, platelet growth factor, pre-eclampsia, sFlt-1
Introduction
Pre-eclampsia, a leading cause of fetal growth restriction, indicated premature delivery and maternal death, affects up to 7% of pregnancies in the USA and is responsible for over 50 000 maternal deaths annually worldwide.1,2 There is currently major interest in developing biomarkers to predict, early in gestation, those women who will develop pre-eclampsia, particularly early-onset or severe pre-eclampsia.
Pre-eclampsia is described as a two-stage phenomenon with abnormal placentation or uteroplacental perfusion leading to increased inflammatory response and endothelial dysfunction giving the systemic maternal syndrome.3 Proposed biomarkers for predicting onset or severity have been those that measure trophoblast invasion, impedance to uteroplacental blood flow, placental function, or production of pro-angiogenic and anti-angiogenic factors. In cross-sectional studies of women with established pre-eclampsia, clear differences have been seen in levels of pro-angiogenic and anti-angiogenic biomarkers.4,5 Although a high degree of sensitivity and specificity for prediction of pre-eclampsia is claimed for first-trimester measurement of biomarkers,6–8 our study in a low-risk nulliparous population9 and a recent systematic review of 37 studies assessing 71 different combinations of biochemical and ultrasonographic markers10 suggest that current first-trimester biomarkers may not have sufficient sensitivity to be clinically useful. Cross-sectional studies of biomarkers in late gestation show more distinct differences between normotensive women and those who will develop pre-eclampsia. Therefore, measuring changes in biomarkers between the first and second trimesters has been proposed as a better early predictor than single first-trimester values.11
The study objective was to test the hypothesis that measurement of change in angiogenic biomarker levels between the first and early or late second trimesters has clinical utility in predicting pre-eclampsia. Although pre-eclampsia occurs at a higher rate in women with pre-existing risk factors,12 this study used low-risk nulliparous women.
Methods
Study design
The Maternal Fetal Medicine Units network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development conducted this study as a planned observational cohort of a larger randomised controlled trial to determine whether antioxidant supplementation (1000 mg vitamin C and 400 IU vitamin E) prevents pre-eclampsia in nulliparous women at low risk for developing the syndrome. Full details of the trial have been reported previously.13 Women were eligible to participate in this cohort if they were enrolled in the trial and their gestational age at enrolment was between 9 weeks 0 days and 12 weeks 6 days. Exclusion criteria were: a previous pregnancy lasting beyond 19 weeks 6 days, an elevated blood pressure (systolic pressure ≥ 135 mmHg or diastolic blood pressure ≥ 85 mmHg), proteinuria (24-hour urine collection of ≥ 300 mg protein or a dipstick value more than trace), current use of antihypertensive medication, pre-gestational diabetes, regular use or use within 7 days of platelet active drugs or nonsteroidal anti-inflammatory agents, known fetal abnormalities or demise, and a history of medical complications. Clinical information including demographic, medical, obstetric, social and sexual history was obtained at the time of enrolment by patient interview and chart review. Blood pressure, weight and urine protein concentrations were recorded at monthly study visits. Blood was collected into EDTA tubes at 9–12, 15–18 and 23–26 weeks of gestation, plasma separated, divided into aliquots and stored at – 70°C until analysis. A nested case–control study was performed with approximately a 3 : 1 random sample of normotensive, nonproteinuric women to women with pre-eclampsia, matched by centre and gestational age at enrolment.
Biochemical assays
We measured platelet growth factor (PlGF), a vascular endothelial gowth factor (VEGF) family member involved in angiogenesis, sFlt, the soluble form of Flt-1 VEGF receptor, and soluble endoglin (sEng), a transforming growth factor-β co-receptor in EDTA plasma. Details of their assay have been reported previously.9 The biomarkers were measured using Luminex assays developed by Rules Based Medicine (Austin, TX, USA). The inter-assay coefficients of variation were 9% at 1.378 ng/ml for sFlt-1, 8% at 3.91 ng/ml for sEng, and 10% at 476 pg/ml for PlGF.
Definitions and outcomes
The primary outcome was the development of pre-eclampsia. Secondary outcomes included the severity and gestational age of pre-eclampsia onset. Mild pre-eclampsia was defined as mild pregnancy-associated hypertension (140–159 mmHg systolic or 90–109 mmHg diastolic on two occasions, 2–240 hours apart) and proteinuria (300–4999 mg total protein/24 hours, 2 + or higher on dipstick testing, or a protein : creatinine ratio of ≥ 0.35). Severe pre-eclampsia was defined as pre-eclampsia with either severe pregnancy-associated hypertension (≥ 160 mmHg systolic or ≥ 110 mmHg diastolic on two occasions, 2–240 hours apart, or a single occurrence treated with anti-hypertensives) or protein excretion of 5 g or more in a 24-hour urine sample or as mild pregnancy-associated hypertension with oliguria (< 500 ml), pulmonary oedema, or thrombocytopenia (platelet count of < 100 000/mm3). Pre-eclampsia included mild and severe pre-eclampsia, HELLP (haemolysis, elevated liver enzymes, low platelet count) syndrome and eclampsia. The time of onset of pre-eclampsia (early onset defined as ≤ 34 weeks or late onset defined as ≥ 34 weeks of gestation) was determined as the time at which individuals first met the criteria for diagnosis of pre-eclampsia given above.
Pre-eclampsia was confirmed via central review, using a standardised protocol by three reviewers not associated with the clinical site of origin, of de-identified medical records of all women with pregnancy-associated hypertension.9
Statistical analysis
Categorical variables were compared using the chi-square test and continuous variables using the Wilcoxon rank sum test. The change in biomarker concentrations between the first (9–12 weeks) and early second (15–18 weeks of gestation) trimesters and between first and late second (23–26 weeks of gestation) trimesters was calculated as rate of change = difference in concentration (pg/ml or ng/ml) divided by number of weeks between measurements. The performance of screening was determined with and without adjustment for significant clinical factors by receiver operating characteristic curves and calculating the sensitivity for a fixed 80% specificity.
Unless specifically noted, a nominal P value less than 0.05 indicated statistical significance and no adjustments were made for multiple comparisons. Analyses were performed using SAS software (SAS Inc., Cary, NC, USA).
Results
Population characteristics
A total of 626 women, 158 diagnosed with pre-eclampsia and 468 normotensive, non-proteinuric, had biomarkers available in both the first trimester and early second trimester. Of these, five delivered before 23 weeks of gestation and 109 did not have a late second trimester blood collection, such that 137 women with pre-eclampsia and 375 normotensive control women were available and used in comparisons of first and late second trimester values. The population characteristics for all women included in the analysis are reported in Table 1. The median gestational age at enrolment was 11.4 weeks (pre-eclampsia) and 11.6 weeks (normotensive controls). Overall there was a significant difference in ethnicity between the women who developed pre-eclampsia and those who remained normotensive. Although there was no difference in the proportion of women who were Hispanic between groups, more women were African American in the pre-eclampsia group with a corresponding reduction in the proportion who were Caucasian. Of those women in this analysis, 13.6% had a family history of pre-eclampsia, 16.8% were smokers and 20.9% had been previously pregnant; there was no difference in these incidences between those who developed pre-eclampsia and those who remained normotensive. However, median body mass index (BMI) and systolic and diastolic blood pressures were significantly greater at enrolment in those women who subsequently developed pre-eclampsia. Patients were equally randomised to vitamins or antioxidants in the clinical trial. The overall incidence of pre-eclampsia among women in this trial was 7.6% and was not different between those who did or did not receive antioxidants.
Table 1.
Population characteristics
| Characteristic | Pre-eclampsia (n = 158) |
No pre-eclampsia (n = 468) |
P-value |
|---|---|---|---|
| Gestational age at sample collection (weeks) | |||
| Enrolment (first-trimester) | 11.4 (10.4–12.3) | 11.6 (10.6–12.3) | 0.62 |
| 15–18 weeks of gestation | 16.6 (16.0–17.4) | 16.4 (16.0–17.0) | 0.06 |
| 23–26 weeks of gestation | 24.7 (24.0–25.7) | 24.7 ( 24.0–25.6) | 0.54 |
| Maternal age (years) | 22 (19–25) | 22 (19–26) | 0.88 |
| Race | 0.019 | ||
| African American | 51 (32.3) | 103 (22.0) | |
| Hispanic | 49 (31.0) | 145 (31.0) | |
| Caucasian/other | 58 (36.7) | 220 (47.0) | |
| Previous pregnancy (< 20 weeks) | 34 (21.5) | 97 (20.7) | 0.83 |
| Family history of pre-eclampsia | 21 (13.3) | 64 (13.7) | 0.90 |
| Smoked during pregnancy | 26 (16.5) | 79 (16.9) | 0.90 |
| BMI at enrolment | 26.7 (22.9–31.6) | 23.5 (21.4–27.6) | < 0.001 |
| Blood pressure at enrolment | |||
| Systolic (mmHg) | 112 (108–120) | 107 (100–115) | < 0.001 |
| Diastolic (mmHg) | 68 (60–72) | 64 (60–70) | < 0.001 |
| Treatment group | 0.79 | ||
| Vitamins C and E | 83 (52.5) | 240 (51.3) | |
| Placebo | 75 (47.8) | 228 (48.7) | |
Data presented as n (%) or median (25–75th centile).
Angiogenic markers
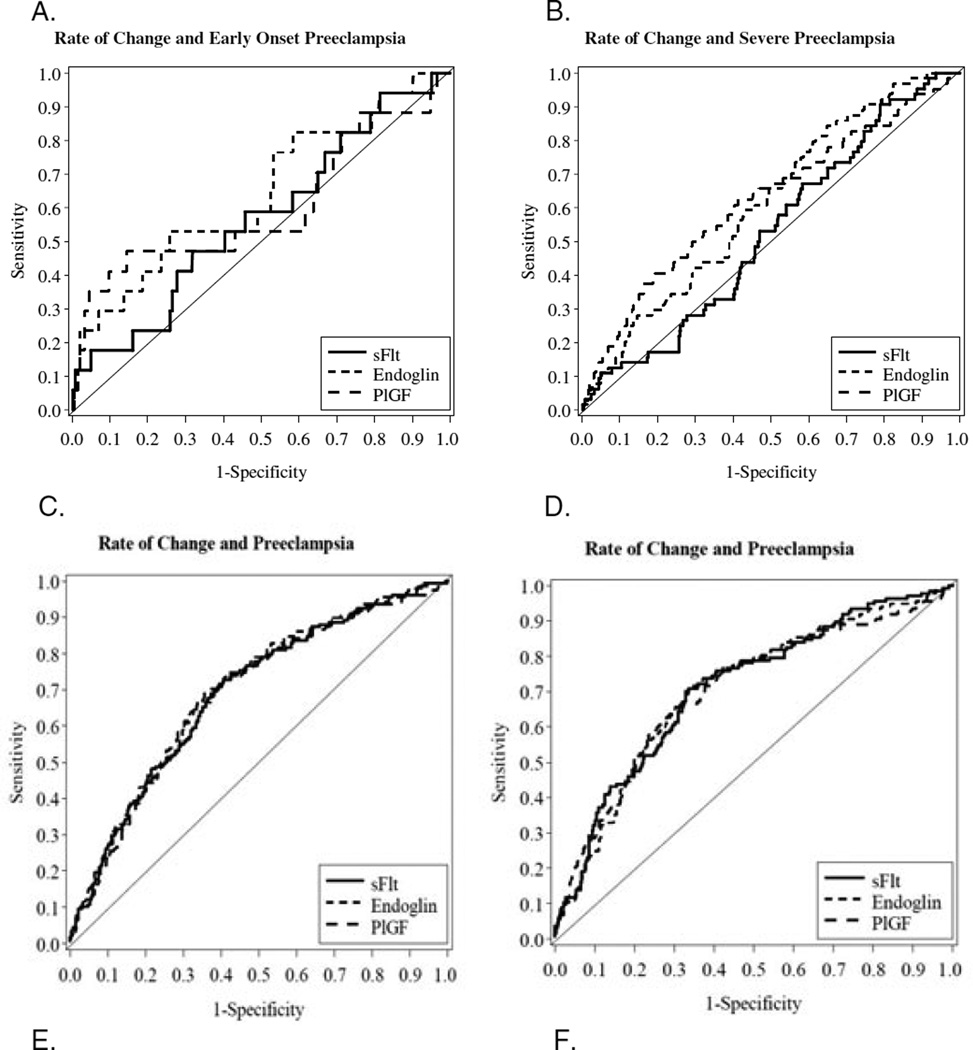
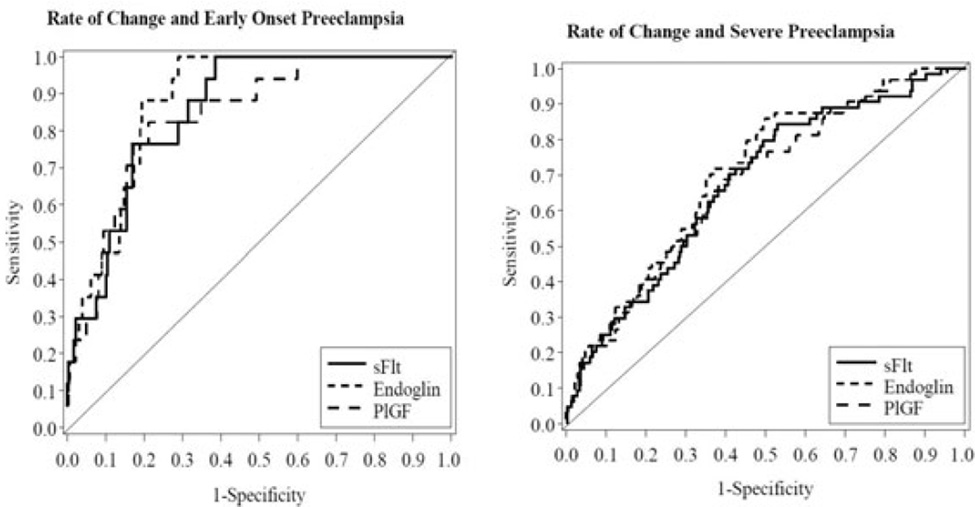
There was no effect of supplementation with vitamins on the concentrations of angiogenic factors. In women who remained normotensive maternal concentrations of both sFlt-1 and PlGF increased from first to early second trimester and from first to to late second trimester (as evidenced by the rate of change, Tables 2 and 3) whereas the concentration of sEng declined slightly over this time. The rates of the change in the biomarkers between first and early second trimesters were significantly different for sEng and PlGF, but not for sFlt-1, between those individuals who developed pre-eclampsia and those who remained normotensive (Table 2). When the different clinical phenotypes of pre-eclampsia were considered the rates of change in sEng and PlGF were again significantly less in those who developed severe pre-eclampsia compared with those who remained normotensive (Table 2). However, only the rate of change for sEng between first and early second trimesters barely reached significance and that for sFlt and PlGF was not significantly different for those who developed early onset pre-eclampsia compared with women who remained normotensive (Table 2). When receiver operating characteristic curves were constructed for the change in biomarkers, the greatest areas under the curve (AUC) were for sEng (0.61, 95% CI 0.54–0.68) and for PlGF (0.62, 95% CI 0.54–0.70) for those women who developed severe pre-eclampsia (Figure 1A) and 0.64 (95% CI 0.49–0.79) and 0.60 (95% CI 0.43–0.77), respectively, for those who developed early onset pre-eclampsia (Figure 1B). The sensitivity of PlGF for predicting early onset pre-eclampsia was 47% (95% CI 23–72%) at an 80% specificity but only 41% (95% CI 29–54%) for prediction of severe pre-eclampsia. When data for change between first trimester and late second trimester were considered, the rate of change for PlGF (reduced), sFlt-1 but not sEng was significantly different in women who developed pre-eclampsia compared with women who remained normotensive (Table 3).
Table 2.
Rate of change in maternal plasma angiogenic factors from first to early second trimester (15–18 weeks of gestation)
| Normotensive (n = 468) |
Pre- eclampsia (n = 158) |
P* | Severe PE (n = 64) |
P** | Early-onset PE (n = 17) |
P*** | |
|---|---|---|---|---|---|---|---|
| sFlt (pg/ml) | |||||||
| First trimester | 1290 (843–1785) | 1140 (793–1740) | 0.14 | 1000 (762–1450) | 0.007 | 958 (762–1680) | 0.19 |
| Second trimester (15–18 weeks) | 1530 (987–2355) | 1340 (841–2170) | 0.07 | 1215 (819–2095) | 0.08 | 1490 (781–2200) | 0.95 |
| Rate of change (pg/ml/week) | 40 (−33 to 148) | 37 (−21 to 133) | 0.78 | 53 (−16 to 136) | 0.52 | 80 (−6 to 143) | 0.35 |
| Endoglin (ng/ml) | |||||||
| First trimester | 4.4 (3.7–5.4) | 4.4 (3.6–5.5) | 0.50 | 4.3 (3.6–5.2) | 0.20 | 3.7 (3.5–4.8) | 0.16 |
| Second trimester (15–18 weeks) | 4.2 (3.4–5.2) | 4.2 (3.5–5.3) | 0.51 | 4.3 (3.5–5.9) | 0.22 | 4.2 (3.4–6.3) | 0.47 |
| Rate of change (ng/ml/week) | −0.06 (−0.23 to −0.09) | −0.02 (−0.15 to −0.15) | 0.036 | 0.01 (−0.11 to 0.22) | 0.004 | 0.09 (−0.08 to 0.38) | 0.05 |
| PlGF (pg/ml) | |||||||
| First trimester | 26.2 (18.6–37.6) | 22.4 (14.8–31.7) | < 0.001 | 22.3 (15.7–27.9) | <0.001 | 26.0 (14.6–42.1) | 0.49 |
| Second trimester (15–18 weeks) | 99.0 (69.7–140.0) | 79.0 (53.1–121.0) | < 0.001 | 73.3 (51.9–112.5) | 0.002 | 85.5 (38.0–122.0) | 0.07 |
| Rate of change (pg/ml/week) | 13.8 (8.8–21.3) | 10.8 (6.5–17.2) | < 0.001 | 9.7 (5.9–17.3) | 0.002 | 12.1 (3.0–18.9) | 0.16 |
PE, pre-eclampsia.
Data presented as median (25–75th centile).
Pre-eclampsia compared with normotensive
Severe preeclampsia compared with normotensive
Early onset preeclampsia compared with normotensive
Table 3.
Rate of change of maternal plasma angiogenic factors from first to late second trimester (23–26 weeks of gestation)
| Normotensive (n = 375) |
Pre- eclampsia (n = 137) |
P* | Severe PE (n = 54) |
P** | Early-onset PE (n = 15) |
P*** | |
|---|---|---|---|---|---|---|---|
| sFlt (pg/ml) | |||||||
| First trimester | 1290 (840–1820) | 1110 (762–1680) | 0.038 | 943 (741–1430) | 0.001 | 949 (625–1450) | 0.038 |
| Second trimester (23–26 weeks) | 1480 (967–2360) | 1570 (924–2570) | 0.59 | 1620 (924–3220) | 0.39 | 1570 (924–4350) | 0.52 |
| Rate of change (pg/ml/week) | 16.4 (−17.9 to 60.6) | 23.1 (−7.8 to 81.0) | 0.044 | 43.0 (7.9–100.1) | <0.001 | 20.6 (1.6–249.2) | 0.09 |
| Endoglin (ng/ml) | |||||||
| First trimester | 4.4 (3.7–5.3) | 4.4 (3.6–5.5) | 0.81 | 4.3 (3.6–5.2) | 0.52 | 3.7 (3.5–4.8) | 0.15 |
| Second trimester (23–26 weeks) | 4.2 (3.6–5.2) | 4.3 (3.7–5.5) | 0.34 | 4.9 (3.9–6.0) | 0.013 | 4.9 (3.4–7.2) | 0.33 |
| Rate of change (ng/ml/week) | −0.007 (−0.09 to 0.05) | −0.004 (−0.08 to 0.06) | 0.20 | 0.032 (−0.03 to 0.09) | 0.002 | 0.02 (−0.02 to 0.18) | 0.06 |
| PlGF (pg/ml) | |||||||
| First trimester | 27.4 (18.6–38.4) | 22.4 (14.8–31.7) | < 0.001 | 22.0 (15.5–27.6) | < 0.001 | 26.0 (14.6–45.1) | 0.63 |
| Second trimester (23–26 weeks) | 360 (252–558) | 240 (151–398) | < 0.001 | 210 (133–366) | < 0.001 | 169 (85–366) | 0.002 |
| Rate of change (pg/ml/week) | 25.6 (16.7–40.4) | 16.8 (9.5–26.6) | < 0.001 | 13.8 (8.9–24.5) | < 0.001 | 9.5 (4.8–25.6) | 0.003 |
PE, pre-eclampsia.
Data presented as median (25–75th centile).
Pre-eclampsia compared with normotensive.
Severe pre-eclampsia compared with normotensive.
Early onset pre-eclampsia compared with normotensive.
Figure 1.
Receiver operating characteristic curves for rate of change in concentration of biomarkers between first and early second trimester for (A) early-onset pre-eclampsia and (B) severe pre-eclampsia, and with the addition of race, body mass index and blood pressure between (C) first and early second trimester for pre-eclampsia, (D) first and late second trimester for pre-eclampsia, (E) first and early second trimester for early-onset pre-eclampsia or (F) first and early second trimester for severe pre-eclampsia.
The rate of change for all three analytes was significantly different in women who developed severe pre-eclampsia compared with women who remained normotensive. Only the rate of change of PlGF was significantly different comparing those who developed early onset pre-eclampsia with those who remained normotensive (Table 3). The predictive performance was reanalysed following addition of the clinical characteristics, race, BMI and blood pressure at enrolment. For changes from first to early second trimester (Table 4) each analyte still only yielded an AUC < 0.70 and a sensitivity < 45% at 80% specificity for predicting pre-eclampsia (Figure 1C,D, Table 4). However, for prediction of early onset pre-eclampsia the AUC for sEng was 0.88 (95% CI 0.83–0.93) yielding a sensitivity of 88.2% (95% CI 63.6–98.5%) at 80% specificity, with performance of sFlt-1 and PlGF only slightly less (Figure 1E, Table 4). The performance for predicting severe pre-eclampsia (Figure 1F, Table 4) was better than that of the biomarkers alone (Figure 1B) but did not match that for prediction of early-onset pre-eclampsia. Similar results were seen for the change from first to late second trimester (Table 5). The sensitivity of PlGF for severe pre-eclampsia reached 60% and the sensitivity of sFlt for prediction of early onset pre-eclampsia now reached 80%.
Table 4.
Area under the curve from receiver operating curves and sensitivity at a fixed 80% specificity for changes in biomarker concentrations adjusted for race, BMI and blood pressure between first and early second trimesters with severe or early-onset pre-eclampsia
| Biomarker (rate of change) |
Pre-eclampsia (n = 158) | Severe pre-eclampsia (n =64) |
Early Onset pre-eclampsia (n = 17) |
|||
|---|---|---|---|---|---|---|
| AUC (95% CI) |
Sensitivity (95% CI) |
AUC (95% CI) |
Sensitivity (95% CI) |
AUC (95% CI) |
Sensitivity (95% CI) |
|
| sFlt | 0.69 (0.64–0.74) | 44.3 (36.4–52.4) | 0.68 (0.61–0.74) | 34.4 (22.9–47.3) | 0.86 (0.79–0.92) | 76.5 (50.1–93.2) |
| Endoglin | 0.69 (0.65–0.74) | 43.7 (35.8–51.8) | 0.70 (0.64–0.77) | 40.6 (28.5–53.6) | 0.88 (0.83–0.93) | 88.2 (63.6–98.5) |
| PlGF | 0.69 (0.64–0.74) | 42.4 (34.6–50.5) | 0.68 (0.61–0.75) | 39.1 (27.1–52.1) | 0.84 (0.76–0.93) | 76.5 (50.1–93.2) |
Table 5.
Area under the curve from receiver operating curves and sensitivity at a fixed 80% specificity for changes in biomarker concentrations adjusted for race, BMI and blood pressure between first and late second trimesters with severe or early-onset pre-eclampsia
| Biomarker (rate of change) |
Pre-eclampsia (n = 137) | Severe pre-eclampsia (n = 54) |
Early-onset pre-eclampsia (n = 15) |
|||
|---|---|---|---|---|---|---|
| AUC (95% CI) |
Sensitivity (95% CI) |
AUC (95% CI) |
Sensitivity (95% CI) |
AUC (95% CI) |
Sensitivity (95% CI) |
|
| sFlt | 0.71 (0.66–0.76) | 47.4 (38.9–56.1) | 0.73 (0.65–0.80) | 57.4 (43.2–70.8) | 0.88 (0.81–0.95) | 80.0 (51.9–95.7) |
| Endoglin | 0.71 (0.66–0.76) | 51.1 (42.4–59.7) | 0.72 (0.65–0.80) | 46.3 (32.6–60.4) | 0.87 (0.81–0.94) | 73.3 (44.9–92.2) |
| PlGF | 0.70 (0.65–0.76) | 48.2 (39.6–56.9) | 0.73 (0.65–0.81) | 59.3 (45.0–72.4) | 0.85 (0.76–0.93) | 73.3 (44.9–92.2) |
Discussion
In this study examining rates of change in pro-angiogenic and anti-angiogenic markers alone from the late first to early second trimester in the low-risk nulliparous population, we find no better sensitivity for predicting pre-eclampsia than our previous analysis of first-trimester markers alone, as ratios or in combination with clinical indices (AUC 0.73 and sensitivity 46% at 20% false-positive rate).9 However, the combination of change in biomarkers with the baseline clinical characteristics of race, BMI and blood pressure increased predictive performance. Performance for pre-eclampsia and severe pre-eclampsia still only reached the levels reported previously using first-trimester biochemical and clinical indices,9 an AUC of 0.88 and sensitivity of 88% for early-onset pre-eclampsia was achieved for change in soluble endoglin between first trimester (median 11 weeks) and early second trimester (15–18 weeks). Measuring change between first and late second trimester with clinical characteristics yielded a slightly lower AUC and sensitivity for detecting early-onset pre-eclampsia with sEng but the performance of all three angiogenic factors for detection of severe pre-eclampsia improved somewhat with PlGF giving an AUC of 0.74 and sensitivity of 59%.
This study is unique as a prospectively designed longitudinal study in a population at low risk for developing pre-eclampsia enrolled in the first trimester. All material was collected and biophysical measurements were performed in a standardised manner by personnel trained in the methodology. Pre-eclampsia was defined according to pre-established criteria and independently reviewed by a panel of three individuals blinded to patient identity. The women were part of a randomised trial of antioxidants.13 We focused on nulliparous women because this group accounts for most cases of pre-eclampsia in clinical practice. We performed by far the largest, comprehensive longitudinal study of trophoblast and angiogenic markers.
Placental growth factor shares homology with VEGF, which can induce proliferation, migration and activation of endothelial cells,14 but which is expressed in villous cytotrophoblast, syncytiotrophoblast and extravillous trophoblast.15,16 Women who subsequently develop pre-eclampsia show reduced PlGF concentrations even in the first trimester and the difference between these women and those who remain normotensive becomes more marked as pregnancy progresses.17 Of the angiogenic factors we have measured in the first trimester, PlGF has the best predictive power but has a low sensitivity.9 Maternal blood concentrations of the anti-angiogenic factors sFlt-1 and sEng, both also produced by trophoblast,18 are low throughout the first and second trimesters and only increase markedly in the third trimester. Previously Vatten et al.19 using a retrospective nested case–control study of women of all parities and Kusanovic et al.20 using a longitudinal cohort approach performed similar studies for PlGF, VEGF, sFlt-1 and sEng reporting that change in concentration of PlGF from first to either early or late second trimester was the best predictor, being significantly different both for all pre-eclampsia and for any of the four clinical phenoytypes, mild, severe, early onset or late onset from women who remained normotensive. We find that the rate of change of PlGF was significantly different for all three phenotypes examined, except for early-onset pre-eclampsia, using data from first to early second trimester. The small number (n = 17) of women with early onset (< 34 weeks of gestation) potentially limited the predictive power of our analysis for this subtype, although we have more cases than Kusanovic et al.20 at < 34 weeks (n = 9) and Vatten et al.19 only studied < 37 weeks of gestation. In contrast to PlGF, the rate of change from first to early second trimester for sEng was significantly different for all pre-eclampsia, severe pre-eclampsia and early-onset pre-eclampsia, but that for sFlt-1 was not related to any subtype. When examining change from first to late second trimester, sEng is only significant for severe pre-eclampsia and sFlt-1 for all pre-eclampsia and severe pre-eclampsia. The sensitivity of such change for severe pre-eclampsia reached 60% for PlGF from first to late second trimester at a fixed 80% specificity. For early-onset pre-eclampsia the sensitivity was better, reaching 73–77% for early and late second time intervals. Earlier studies had also shown that a combination of the slopes of PlGF and sEng gave the best predictive value particularly for early-onset pre-eclampsia.19,20 Indeed the study by Kusanovic et al.20 claimed a sensitivity of 100% with a specificity of 98–99% for prediction of early-onset pre-eclampsia using the PlGF : sEng ratio. Determining the slopes of the PlGF : sFlt or PlGF : sEng ratios did not improve sensitivity above that of PlGF alone (data not shown).
Conclusion
In contrast to previous reports we do not find that assessment of changes in angiogenic markers alone from first to second trimester can improve predictive power. However, addition of baseline clinical characteristics improved the predictive power for severe and particularly for early-onset pre-eclampsia such that sensitivity reached 88% at an 80% specificity. This differential sensitivity for detection of early-onset pre-eclampsia reinforces the idea that the there may be more than one pathophysiological phenotype.21 As the utility of therapeutic intervention to change the pathological course or of surveillance would be enhanced by prediction as early as possible in gestation, measurement of change between first trimester (median 11 weeks) and early second trimester (15–18 weeks) appears to have the most value, particularly as the test has highest sensitivity for early-onset pre-eclampsia, the group with shortest time available for intervention. Future studies should focus on the utility of combinations of biomarkers and clinical factors in prediction of different clinical phenotypes of pre-eclampsia.
Acknowledgements
The authors thank the following Subcommittee members who participated in protocol development and coordination between clinical research centres (Sabine Bousleiman and Margaret Cotroneo), protocol development and statistical analysis (Elizabeth Thom) and protocol development and oversight (John C. Hauth, Kenneth J. Leveno and Gail D. Pearson). In addition to the authors, other members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network are as follows:
University of Pittsburgh, Pittsburgh, PA—S. Caritis, T. Kamon (deceased), M. Cotroneo, D. Fischer
University of Utah, Salt Lake City, UT—M. Varner, P. Reed, S. Quinn (LDS Hospital), V. Morby (McKay-Dee Hospital), F. Porter (LDS Hospital), R. Silver, J. Miller (Utah Valley Regional Medical Center), K. Hill
University of Alabama at Birmingham, Birmingham, AL—J. Hauth, D.J. Rouse, A. Northen, P. Files, J. Grant, M. Wallace, K. Bailey
Columbia University, New York, NY—R. Wapner, S. Bousleiman, R. Alcon, K. Saravia, F. Loffredo, A. Bayless (Christiana), C. Perez (St. Peter's University Hospital), M. Lake (St. Peter's University Hospital), M. Talucci
University of North Carolina at Chapel Hill, Chapel Hill, NC—K. Boggess, K. Dorman, J. Mitchell, K. Clark, S. Timlin
Case Western Reserve University-MetroHealth Medical Center, Cleveland, OH—J. Bailit, C. Milluzzi, W. Dalton, C. Brezine, D. Bazzo
University of Texas Southwestern Medical Center, Dallas, TX—K. Leveno, J. Sheffield, L. Moseley, M. Santillan, K. Buentipo, J. Price, L. Sherman, C. Melton, Y. Gloria-McCutchen, B. Espino
Northwestern University, Chicago, IL—M. Dinsmoor (NorthShore University HealthSystem), T. Matson-Manning, G. Mallett
University of Texas Health Science Center at Houston, Houston, TX—S. Blackwell, K. Cannon, S. Lege-Humbert, Z. Spears
Brown University, Providence, RI—J. Tillinghast, M. Seebeck
The Ohio State University, Columbus, OH—P. Samuels, F. Johnson, S. Fyffe, C. Latimer, S. Frantz, S. Wylie, J. Iams
Drexel University, Philadelphia, PA—M. Talucci, M. Hoffman (Christiana), J. Benson (Christiana), Z. Reid, C. Tocci
Wake Forest University Health Sciences, Winston-Salem, NC—M. Harper, P. Meis, M. Swain
Oregon Health & Science University, Portland, OR—W. Smith, L. Davis, E. Lairson, S. Butcher, S. Maxwell, D. Fisher
University of Texas Medical Branch, Galveston, TX—J. Moss, B. Stratton, G. Hankins, J. Brandon, C. Nelson-Becker, G. Olson, L. Pacheco
Wayne State University, Detroit, MI—G. Norman, S. Blackwell, P. Lockhart, D. Driscoll, M. Dombrowski
The George Washington University Biostatistics Center, Washington, DC—E. Thom, T. Boekhoudt, L. Leuchtenburg
National Heart, Lung, and Blood Institute, Bethesda, MD—G. Pearson, V. Pemberton, J. Cutler, W. Barouch
Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD—S. Tolivaisa.
Funding
The project described was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (HD34208, HD27869, HD40485, HD40560, HD40544, HD34116, HD40512, HD21410, HD40545, HD40500, HD27915, HD34136, HD27860, HD53118, HD53097, HD27917, and HD36801); the National Heart, Lung, and Blood Institute; and the National Center for Research Resources (M01 RR00080, UL1 RR024153, UL1 RR024989) and its contents do not necessarily represent the official view of NICHD, NHLBI, NCRR or NIH.
Footnotes
This work was presented at the XVII Meeting International Society for the Study of Hypertension in Pregnancy, Melbourne, Australia: Changes in Plasma Angiogenic/Antiangiogenic Proteins between First and Second Trimester for Prediction of Preeclampsia in a Low Risk Population, 3–6 October 2010.
Disclosure of interests
None of the authors have a conflict of interest.
Contribution to authorship
LM and JMR conceived the study. All authors (LM, RGC, JMR, CYS, RJW, JMT, BMM, AMP, SMM, MWC, AS, JET, GS, YS and GDA) were involved in planning and carrying out the study. LM and RGC analysed the data and wrote the initial drafts of the manuscript. All authors (LM, RGC, JMR, CYS, RJW, JMT, BMM, AMP, SMM, MWC, AS, JET, GS, YS and GDA) reviewed, edited and approved the final submitted version.
Details of ethics approval
Written informed consent was obtained from every woman and the study was approved by the institutional review board at each clinical site and the data-coordinating centre.
References
- 1.World Health Organization. Estimates of maternal mortality: a new approach by WHO and UNICED. Geneva: World Health Organization; 1996. [Google Scholar]
- 2.Which anticonvulsant for women with eclampsia? Evidence from the Collaborative Eclampsia Trial. Lancet. 1995;345:1455–1463. [PubMed] [Google Scholar]
- 3.Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta. 2009;30(Suppl A):S32–S37. doi: 10.1016/j.placenta.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 5.Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008;21:9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laigaard J, Sorensen T, Placing S, Holck P, Frohlich C, Wojdemann KR, et al. Reduction of the disintegrin and metalloprotease ADAM12 in preeclampsia. Obstet Gynecol. 2005;106:144–149. doi: 10.1097/01.AOG.0000165829.65319.65. [DOI] [PubMed] [Google Scholar]
- 7.Poon LC, Akolekar R, Lachmann R, Beta J, Nicoaides KH. Hypertensive disorders in pregnancy: screening by biophysical and biochemical markers at 11–13 weeks. Ultrasound Obstet Gynecol. 2010;35:662–670. doi: 10.1002/uog.7628. [DOI] [PubMed] [Google Scholar]
- 8.Chafetz I, Kuhnreich I, Sammar M, Tal Y, Gibor Y, Meiri H, et al. First-trimester placental protein 13 screening for preeclampsia and intrauterine growth restriction. Am J Obstet Gynecol. 2007;197:35, e1–e7. doi: 10.1016/j.ajog.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 9.Myatt L, Clifton RG, Roberts JM, Spong CY, Hauth JC, Varner MW, et al. First-trimester prediction of preeclampsia in nulliparous women at low risk. Obstet Gynecol. 2012;119:1234–1242. doi: 10.1097/AOG.0b013e3182571669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giguere Y, Charland M, Bujold E, Bernard N, Grenier S, Rousseau F, et al. Combining biochemical and ultrasonographic markers in predicting preeclampsia: a systematic review. Clin Chem. 2010;56:361–375. doi: 10.1373/clinchem.2009.134080. [DOI] [PubMed] [Google Scholar]
- 11.Erez O, Romero R, Espinoza J, Fu W, Toden D, Kusanovic JP, et al. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med. 2008;21:279–287. doi: 10.1080/14767050802034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caritis S, Sibai B, Hauth J, Lindheimer MD, Klebanoff M, Thorn E, et al. Low-dose aspirin to prevent preeclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998;338:701–705. doi: 10.1056/NEJM199803123381101. [DOI] [PubMed] [Google Scholar]
- 13.Roberts JM, Myatt L, Spong CY, Thorn EA, Hauth JC, Leveno KJ, et al. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010;362:1282–1291. doi: 10.1056/NEJMoa0908056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ziche M, Maglione D, Ribatti D, Morbidelli L, Lago CT, Battisti M, et al. Placenta growth factor-1 is chemotactic, mitogenic, and angiogenic. Lab Invest. 1997;76:517–531. [PubMed] [Google Scholar]
- 15.Clark DE, Smith SK, Licence D, Evans AL, Charnock-Jones DS. Comparison of expression patterns for placenta growth factor, vascular endothelial growth factor (VEGF), VEGF-B and VEGF-C in the human placenta throughout gestation. J Endocrinol. 1998;159:459–467. doi: 10.1677/joe.0.1590459. [DOI] [PubMed] [Google Scholar]
- 16.Khaliq A, Li XF, Shams M, Sisi P, Acevedo CA, Whittle MJ, et al. Localisation of placenta growth factor (PIGF) in human term placenta. Growth Factors. 1996;13:243–250. doi: 10.3109/08977199609003225. color plates I-II,pre bk cov. [DOI] [PubMed] [Google Scholar]
- 17.Tidwell SC, Ho HN, Chiu WH, Torry RJ, Torry DS. Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. Am J Obstet Gynecol. 2001;184:1267–1272. doi: 10.1067/mob.2001.113129. [DOI] [PubMed] [Google Scholar]
- 18.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vatten LJ, Eskild A, Nilsen TI, Jeansson S, Jenum PE, Staff AC, et al. Changes in circulating level of angiogenic factors from the first to second trimester as predictors of preeclampsia. Am J Obstet Gynecol. 2007;196:239, e1–e6. doi: 10.1016/j.ajog.2006.10.909. [DOI] [PubMed] [Google Scholar]
- 20.Kusanovic JP, Romero R, Chaiworapongsa T, Erez O, Mittal B, Vaisbuch E, et al. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med. 2009;22:1021–1038. doi: 10.3109/14767050902994754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myatt L, Carpenter L. Prediction of Preeclampsia. In: Lyall F, Belfort M, editors. Preeclampsia Etiology and Clinincal Practice. Cambridge: Cambridge University Press; 2007. [Google Scholar]