Abstract
Background
Achallenge for emergency medical service (EMS) is accurate identification of acute coronary syndromes (ACS) and ST elevation myocardial infarction (STEMI) for immediate treatment and transport. The electrocardiograph-based acute cardiac ischemia time-insensitive predictive instrument (ACI-TIPI) and the thrombolytic predictive instrument (TPI) have been shown to improve diagnosis and treatment in emergency departments (EDs), but their use by paramedics in the community has been less studied.
Methods
Ambulances in study municipalities were outfitted with electrocardiographs with ACI-TIPI and TPI software. Using a before-after quasi-experimental design, in Phase 1, for seven months, paramedics were provided with the ACI-TIPI/TPI continuous 0–100% predictions automatically printed on electrocardiogram (ECG) text headers to supplement their identification of ACS; in Phase 2, for 11 months, paramedics were told to identify ACS based on an ACI-TIPI cutoff probability of ACS ≥ 75% and/or TPI detection of STEMI. In Phase 3, this cutoff approach was used in seven additional municipalities. Confirmed diagnoses of ACS, acute myocardial infarction (AMI), and STEMI were made by blinded physician review for 100% of patients.
Results
In Phase 1, paramedics identified 107 patients as having ACS; in Phase 2, 104. In Phase 1, 45.8% (49) of patients so-identified had ACS confirmed, which increased to 76.0% (79) in Phase 2 (p < 0.001). Of those with ACS, in Phase 1 59.2% (29) had AMI versus 84.8% (67) with AMI in Phase 2 (p <0.01), and, STEMI was confirmed, respectively, in 40.8% (20), versus 68.4% (54) (p <0.01). In Phase 3, of 226 patients identified by paramedics as having ACS, 74.3% (168) had ACS confirmed, of whom 81.0% (136) had AMI and 65.5% (110) had STEMI.
Conclusions
In a wide range of EMS systems, use of electrocardiographs with ACI-TIPI and TPI decision support using a 75% ACI-TIPI cutoff improves paramedic diagnostic performance for ACS, AMI, and STEMI.
Keywords: acute coronary syndromes, acute myocardial infarction, electrocardiology, emergency medical service, clinical decision support
BACKGROUND
For patients in the community with symptoms suggestive of acute coronary syndromes (ACS), rapid and accurate emergency medical service (EMS) evaluation, treatment, and transport are critical, especially for patients with ST elevation myocardial infarction (STEMI). These functions, and notification of receiving hospitals, have been shown to be improved by paramedic use of prehospital 12-lead electrocardiographs.1–5 However, these results may not apply to EMS systems without paramedics highly-trained in electrocardiogram (ECG) interpretation. To optimize the impact of out-of-hospital ECGs, an approach is needed to identify ACS and STEMI that is effective and applicable across a wide range of communities and EMS systems.
Of patients calling 9-1-1 or arriving at an emergency department (ED) for chest pain or related symptoms, only about 25% will prove to have ACS. Of those with ACS, about two-thirds will prove to have unstable angina pectoris and one-third will prove to have acute myocardial infarction (AMI), of whom, about 40% will have STEMI. Thus, about the task of identifying STEMI is that this represents only 3–5% of all patients presenting with potential cardiac symptoms.5–7 Even for a physician with the diagnostic facilities of the hospital, it is challenging to promptly and accurately identify patients who truly have ACS; it is even more challenging for the paramedic in an ambulance in the community. Yet the opportunity for impact by EMS depends on early identification by paramedics of these patients to expedite and improve treatment for ACS and coronary reperfusion for STEMI.
Reports from highly-effective EMS systems5–7 have shown prehospital ECG diagnostic performance sufficient to allow two important improvements in care for STEMI: 1) transport of patients directly to percutaneous coronary intervention (PCI)-capable facilities with bypass of non-PCI facilities, and 2) activation of PCI lab teams prior to patient arrival, to reduce delays to reperfusion. These directly link to improved patient outcomes and they also help avoid potentially longer transport to PCI facilities unless necessary. However, these reports do not reflect easily applied approaches that can immediately be used in the thousands of EMS systems in this country. Augmenting out-of-hospital diagnosis by electronic transmission of ECGs from the field for medical supervision has been suggested, but such systems are expensive, complex, subject to technical challenges, and their impact has not yet been demonstrated. In the ED, decision support provided by the ECG-based acute cardiac ischemia time-insensitive predictive instrument (ACI-TIPI) and thrombolytic predictive instrument (TPI) has been shown to improve the speed and accuracy of the diagnosis, triage, and treatment of ACS and STEMI.8,9 They have been proposed for EMS use,9,10 but heretofore, they had not been directly tested in the EMS setting.
The IMMEDIATE (Immediate Myocardial Metabolic Enhancement During Initial Assessment and Treatment in Emergency Care) Trial is a National Institutes of Health (NIH) multi-center randomized placebo-controlled effectiveness trial evaluating use of EMS-administered intravenous glucose, insulin and potassium (GIK) for patients with ACS (www.immediatetrial.com). To optimally identify candidates for GIK in this trial, we implemented and assessed EMS paramedic use of the ACI-TIPI and TPI in out-of-hospital electrocardiographs, and evaluated its impact on paramedic on-site identification of ACS and STEMI as a community-based approach to improving emergency cardiac care.
METHODS
Sites
Participating sites included, starting in Winter, 2006, Dallas, TX; Milwaukee, WI; and Brockton and Concord, MA; then in Spring through Fall, 2008, included were Albuquerque, NM; Anchorage, AK; Bellingham, WA; El Paso, TX; Macon, GA; New Haven, CT; and Sioux Falls, SD. At each site, EMS and hospital institutional review board, and community-based approvals were obtained for human subject research and all patients provided written informed consent.
Enrollment criteria
Eligible for enrollment were all patients ≥ 30 years old with symptoms consistent with ACS for which the paramedic performed a 12-lead ECG. Excluded were those who were hemodynamically unstable, or with heart failure (Killip Classes 3 or 4) or end-stage renal failure requiring dialysis.
Phases
The study was conducted in three phases. In the first phase, to identify patients with ACS, paramedics received extensive training in ECG interpretation and the conventional use of the ACI-TIPI and TPI predictions as printed on the ECG header. In the second phase, paramedics were taught to identify patients with ACS based on a new approach to the use of the ACI-TIPI and TPI, designed for EMS use. The third phase consisted of testing this same approach in additional communities. Accordingly, paramedics were instructed to identify patients with ACS and STEMI as follows:
In Phase 1
Following education and hands-on training in ECG interpretation and use of ACI-TIPI and TPI, paramedics were instructed to use their judgment as to whether the patient appeared to be having ACS based on the presenting clinical picture and ECG abnormalities, including use of ACI-TIPI and TPI predictions automatically printed on the ECGs, looking for one of the following in two or more contiguous leads:
ST elevation ≥ 1mm, and/or local STEMI criteria for EMS notification to receiving hospitals for rapid access to a PCI laboratory
ST depression ≥ 0.5mm
T wave inversion or other T wave abnormalities (e.g., hyperacute T waves)
Left bundle branch block not known to be old
In Phases 2 and 3
Paramedics were instructed, if associated with symptoms consistent with ACS, to consider a patient as having ACS or STEMI if one of the following were present:
ACI-TIPI prediction of 75% or more, indicating a very high likelihood of ACS
TPI predictions printed on the ECG, indicating the electrocardiograph detected STEMI
Neither of the above, but STEMI on the ECG as would be used in that EMS system to notify receiving hospitals for rapid access to a PCI laboratory
Training
During Phase 1, paramedics underwent extensive training in ECG interpretation, designed by the IMMEDIATE Trial’s Training Committee, comprised of investigators, EMS medical directors, educators, and paramedics. Prior to the Trial, all participating EMS systems’ paramedics had been performing ECGs in the field for at least two years and all had undergone various ECG interpretation training programs. The IMMEDIATE Trial program started with a 20-question on-line ECG Interpretation Assessment Test to identify the 12-lead ECG reading skill levels and general strengths and weaknesses. Training focused on the accurate identification of ACS and STEMI based on clinical and ECG criteria, including extensive hands-on training. Information from the ECG Assessment Test allowed EMS systems to conduct targeted training on 12-lead ECG interpretation, and re-training, as necessary. Each paramedic was required to complete two ECG modules (totaling four hours): one focused on basic ECG changes (i.e., ST, T wave elevation and depression), and the other focused on ST change “mimics” (i.e., bundle branch blocks, left ventricular hypertrophy, early repolarization). Upon completion, paramedics were required to re-take the ECG Assessment Test to determine improvement and identify new areas for focus. The ECG assessment was comprised of several hundred ECGs, each categorized as having ST or T wave elevation or depression, or having a specific mimic. The online system randomly selected ECGs for a given test to help assure that the paramedics took different tests pre- and post-training. Each paramedic also was required to complete other modules with review tests, including Use of Predictive Instrument Technology (the ACI-TIPI and TPI decision support software made available for Physio-Control Lifepak-12, Zoll M-Series, and Philips MRx electrocardiographs). These also included a hands-on practicum requiring the paramedics to demonstrate their use of the ACI-TIPI and TPI, and their ability to identify patients with ACS or STEMI for eligibility for the Trial. In Phase 1, this included their clinical and ECG assessment and the use of the ACI-TIPI as a 0–100% scale and the TPI to supplement, but not dictate, their decision.
During Phase 2, paramedics underwent additional training to reflect the change in identification of ACS and STEMI to be based on the ACI-TIPI 75% cutoff requirement or the presence of TPI detection of STEMI. This was conducted as part of refresher training requiring only 15 minutes, and posters were placed in key places as a reminder.
In Phase 3, the training that took place at the seven new sites did not include any of the training in ECG interpretation done in Phase 1. Paramedics were only trained in the Use of Predictive Instrument Technology (the ACI-TIPI and TPI decision support software made) that now included the ACI-TIPI 75% cutoff requirement and screening process. This training took 1.5 hours.
In all phases, each paramedic’s performance was monitored using the IMMEDIATE Trial’s real-time reporting system capability to provide ongoing feedback to the paramedics regarding their overall performance.
Development of ACI-TIPI/TPI Threshold for EMS Use
As the basis for the new EMS approach to identifying ACS and STEMI, various ACI-TIPI cutoff thresholds were explored by analyses of databases of EMS and ED patients and in discussions among IMMEDIATE Trial investigators and sites. Specifically, data from the ACI-TIPI Trial,8 TPI Trial,9 and IMMEDIATE Trial ECG database (then including over 3,000 screened subjects), were analyzed for ACI-TIPI and TPI enrollment criteria that could achieve the Trial’s goal of enrolling a cohort of patients with no more than 25% who did not have ACS (i.e., the false positive rate for ACS had to be no higher than 25%). Analyses of the above databases suggested that this could be achieved by including only patients who had ACI-TIPI probabilities of ACS ≥ 75% and/or had a STEMI detected, as evidenced by TPI text being printed. Therefore these ACI-TIPI and TPI thresholds were applied in the second phase of this study.
Study Design
The quasi-experimental before-after assessment of the use of the ACI-TIPI cutoff approach was done in three phases. In Phase 1, in the four EMS systems that started in 2006 or 2007, the paramedics used the ACI-TIPI conventionally, using its 0–100% prediction of ACS to supplement their decision-making, and their correct identification of ACS, AMI, and STEMI were measured. In Phase 2, starting July 1, 2008, the ACI-TIPI 75% with TPI cutoff approach was implemented, and performance was assessed in the same four EMS systems. In Phase 3, the same ACI-TIPI cutoff and TPI approach was implemented in seven new EMS systems, to test performance in a yet wider variety of systems and municipalities. In all cases, the diagnoses assigned by paramedics in the field were compared to “gold standard” diagnoses assigned by physicians, based on clinical records, ECGs, and biomarkers (CK and troponin), using previously validated methods.8
Data Collection
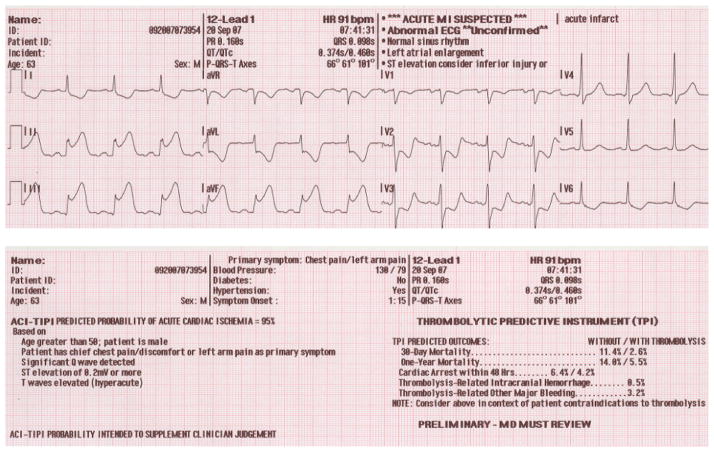
All subjects enrolled in the IMMEDIATE Trial from its onset December 1, 2006 through July 31, 2009 were included in this analysis. Collected data included demographic and clinical information from medical records, ACI-TIPI and TPI predictions from ECG text headers (Fig. 1), and ECGs downloaded from out-of-hospital electrocardiographs.
Figure 1.
Example of Prehospital ACI-TIPI/TPI Electrocardiogram
Analysis
Subjects were classified by the study phase during which they were enrolled. Characteristics were compared between subjects in Phases 1 and 2 using student t-tests (mean age, systolic blood pressure, heart rate, ACI-TIPI score), chi-square tests (gender, ethnicity, medical history, primary symptom of chest pain, TPI triggered on ECG), or Fisher’s exact tests (race).
The ACI-TIPI cutoff was based on analyses of data from the ACI-TIPI Trial8 and the TPI Trial9 and the IMMEDIATE Trial ECG database. Potential combinations of ACI-TIPI score cutoffs and the TPI trigger were reviewed by the investigators in terms of their sensitivity, specificity, and positive and negative predictive values. Based on review of these analyses and performance characteristics of the published models, patients were considered sufficiently likely to be having ACS to warrant treatment for ACS/STEMI if their ACI-TIPI probability of ACS was at least 75% and/or the TPI print-out was triggered by the electrocardiograph’s detection of STEMI.
Diagnoses were assigned by the site physicians (who were blinded to study group assignment) on the basis of presentation, clinical course, and initial and follow-up electrocardiograms and tests for creatine kinase-MB and troponin, using World Health Organization and Canadian Cardiovascular Society criteria.11,12 ACS was defined as being either AMI (including STEMI) or unstable angina.
Study outcomes included the percent of patients identified by paramedics as having ACS who truly had ACS, i.e., patients who were “true positives,” and the proportions who had AMI and STEMI. These proportions, and the proportion of patients identified by paramedics as having ACS who proved not to have ACS, i.e., patients who were “false positives,” were compared between Phases 1 and 2, adjusting for sites using the Cochran-Mantel-Haenzel test. Phase 3 characteristics and outcomes, while also presented, were not compared statistically with data from the other Phases because there was no pre-intervention group at these sites to compare with the post-intervention group and thus site adjustment was not possible.
All presented p-values are from two-sided tests, and considered statistically significant if ≤ 0.05. Analyses were done using SAS for Windows version 9.2. (Cary, North Carolina)
RESULTS
Between December 1, 2006, and July 31, 2009, the 11 participating EMS systems (Table 1), enrolled 437 subjects (Table 2). In Phase 1, 107 subjects were enrolled at four sites: Brockton, MA; Concord, MA; Dallas, TX; and Milwaukee, WI. In Phase 2, 104 subjects were enrolled at these same sites. In Phase 3, 226 were enrolled at seven new sites: Albuquerque, NM; Anchorage, AK; Bellingham, WA; El Paso, TX; Macon, GA; New Haven, CT; and Sioux Falls, SD.
Table 1.
Site Characteristics
| Site Name | Type of EMS System | Population Community Served | Number of Paramedics | Number of ALS Vehicles | Number of Receiving Hospitals |
|---|---|---|---|---|---|
| Brockton MA | Private | 110,000 | 50 | 14 | 2 |
| Concord MA | Hospital | 63,306 | 20 | 2 | 2 |
| Dallas TX | Fire | 1,200,000 | 1100 | 60 | 13 |
| Milwaukee WI | Fire | 604, 477 | 350 | 20 | 3 |
| Albuquerque NM | Private and Fire | 1,166,096 | 121 | 54 | 5 |
| Bellingham WA | Fire | 166,814 | 30 | 8 | 1 |
| New Haven CT | Private and Fire | 408,288 | 210 | 59 | 2 |
| Macon GA | Hospital | 293,447 | 70 | 21 | 1 |
| Sioux Falls SD | Private | 158,424 | 27 | 8 | 3 |
| El Paso TX | Fire | 676,365 | 126 | 20 | 6 |
| Anchorage AK | Fire | 258,455 | 50 | 16 | 2 |
Table 2.
Subject Enrollment by Site and Phase (N=437)
| EMS Sites* | Phase 1 (n=107) | Phase 2 (n=104) | Phase 3 (n=226) |
|---|---|---|---|
| A | 36 | 50 | |
| B | 29 | 30 | |
| C | 33 | 12 | |
| D | 9 | 12 | |
| E | 61 | ||
| F | 35 | ||
| G | 34 | ||
| H | 25 | ||
| I | 26 | ||
| J | 23 | ||
| K | 22 |
Actual sites in Table 1; masked here for confidentiality
Subjects were similar across phases (Table 3), but consistent with the intended change to have higher proportions with ACS, the mean ACI-TIPI probability of ACS significantly increased from 48% in Phase 1 to 70% in Phase 2 (p<0.0001). Also, the proportion of patients who met the ACI-TIPI/TPI cutoff criteria increased from 28% in Phase 1 to 73% in Phase 2. This proportion increased to 81% in Phase 3. This also was reflected by the enrollment of more males in Phase 2 than Phase 1 (82% vs. 56%, p<0.001) and more heart failure in Phase 2 than Phase 1 (26% vs. 13%, p=0.02). As the intended outcome, the proportion of subjects with confirmed ACS, was significantly higher in Phase 2 than Phase 1 (76% vs. 46%, p<0.001), and, shown in Table 4, among those with ACS, the percentages with AMI and STEMI were significantly higher in Phase 2 than Phase 1. Using the same intervention as in Phase 2, the proportion of subjects with confirmed ACS in Phase 3 (74%) was similar to that seen in Phase 2, as were the proportions of those who had AMI and STEMI.
Table 3.
Subject Characteristics Stratified by Enrollment Phase (N=437)
| Characteristic* | Phase 1 (n=107) | Phase 2 (n=104) | P-value for Phases 1 vs. 2† | Phase 3 (n=226) |
|---|---|---|---|---|
| Age (years) | 60.9 ± 15.5 | 62.1 ± 13.5 | 0.54 | 63.5 ± 13.6 |
| Male gender | 56.1% (60) | 81.7% (85) | <0.0001 | 71.2% (161) |
| Hispanic ethnicity | 3.7% (4) | 4.8% (5) | 0.70 | 17.0% (38) |
| Race | 0.53 | |||
| White | 72.0% (77) | 68.3% (71) | 77.4% (175) | |
| Black | 20.6% (22) | 22.1% (23) | 8.4% (19) | |
| Other | 3.7% (4) | 1.9% (2) | 4.4% (10) | |
| Unknown | 3.7% (4) | 7.7% (8) | 9.7% (22) | |
| Medical History | ||||
| Myocardial infarction | 23.4% (25) | 35.6% (37) | 0.06 | 35.8% (81) |
| Coronary artery bypass graft | 15.9% (17) | 15.4% (16) | 0.92 | 17.3% (39) |
| Congestive heart failure | 13.1% (14) | 26.0% (27) | 0.02 | 15.0% (34) |
| Percutaneous coronary intervention (PCI) | 21.5% (23) | 24.0% (25) | 0.66 | 34.1% (77) |
| Angina | 21.5% (23) | 14.4% (15) | 0.18 | 8.8% (20) |
| Arrhythmia | 24.3% (26) | 22.1% (23) | 0.71 | 18.1% (41) |
| Diabetes | 29.0% (31) | 28.8% (30) | 0.99 | 26.5% (60) |
| Hypertension | 62.6% (67) | 63.5% (66) | 0.90 | 68.6% (155) |
| Hypercholesterolemia | 48.6% (52) | 37.5% (39) | 0.11 | 51.8% (117) |
| Stroke | 6.5% (7) | 8.7% (9) | 0.57 | 8.4% (19) |
| EMS Data | ||||
| Primary symptom chest pain | 77.6% (83) | 85.6% (89) | 0.14 | 88.5% (200) |
| Systolic blood pressure (mmHg) | 145.2 ± 31.3 | 147.7± 35.2 | 0.59 | 142.2 ± 33.8 |
| Heart rate (bpm) | 91.6 ± 24.9 | 86.1 ± 23.4 | 0.11 | 85.6 ± 25.3 |
| ACI-TIPI (% prediction of ACS) | 48.0 ± 26.2 | 70.5 ± 22.7 | <0.0001 | 79.4 ± 17.3 |
| TPI trigger | 17.0% (18) | 47.1% (49) | <0.0001 | 37.2% (84) |
| ACI-TIPI ≥ 75% or TPI triggered | 27.6% (29) | 72.5% (74) | <0.0001 | 81.2% (181) |
Data are presented as means ± standard deviations (age, blood pressure, heart rate, ACI-TIPI score) or percent (sample size) for all other characteristics. There were missing data for some characteristics. Specifically, ethnicity data were missing for two subjects (Phase 3). Systolic blood pressure, heart rate and the ACI-TIPI score data were missing for less than 3% of subjects. Data needed to determine if either TIPI ≥ 75 or TPI trigger was true were missing for seven subjects (two in Phase 1, two in Phase 2, and three in Phase 3).
P-values are from chi-square test (gender, ethnicity, site), Fisher’s exact test (race), or t-test (age) used to compare characteristics between Phase 1 and Phase 2.
Table 4.
Diagnosis and Treatment Outcomes (N=437 total enrolled)
| Confirmed Diagnosis | Phase 1 | Phase 2 | P-value for Phases 1 vs. 2* | Phase 3 |
|---|---|---|---|---|
| All enrolled subjects | N=107 | N=104 | N=226 | |
| Diagnosis of ACS (True positives), % of total | 45.8% (49) | 76.0% (79) | <0.0001 | 74.3% (168) |
| Diagnosis of AMI, % of total | 27.1% (29) | 64.4% (67) | <0.0001 | 60.2% (136) |
| Diagnosis of STEMI, % of total | 18.7% (20) | 51.9% (54) | <0.0001 | 48.7% (110) |
| Subset of Enrolled Subjects with ACS | N=49 | N=79 | N=168 | |
| Diagnosis of AMI, % of ACS | 59.2% (29) | 84.8% (67) | 0.001 | 81.0% (136) |
| Diagnosis of STEMI, % of subset | 40.8% (20) | 68.4% (54) | 0.002 | 65.5% (110) |
| Subset of Enrolled Subjects with STEMI | N=20 | N=54 | N=110 | |
| PCI, % of subset | 75.0% (15) | 83.3% (45) | 0.4 | 82.7% (91) |
P-values come from the Cochran-Mantel-Haenzel test for general association controlling for site comparing confirmed diagnosis percentages between Phase 1 and Phase 2. Phase by site interactions were tested using the Breslow-Day test for homogeneity of odds ratios for each confirmed diagnosis outcome with none reaching significance (p>.10 for all outcomes).
To further explore the potential impact of the ACI-TIPI/TPI cutoff instructions, retrospectively, among the patients so-identified by paramedics, the percentages of patients with confirmed ACS, AMI, and STEMI were compared between subjects who actually met the ACI-TIPI/TPI cutoff and those who did not. As shown in Table 5, the proportion of subjects with ACS was significantly higher among those who met the ACI-TIPI/TPI cutoff than those who did not (79.6% vs. 45.2%, p <0.0001), as were the proportions of those with ACS who had AMI (85.8% vs. 53.0%, p<0.0001) and who had STEMI (73.0% vs. 25.8%, p<0.0001).
Table 5.
Confirmed Diagnoses of Patients Based on Retrospective Classification of Meeting Cutoff-based Acute Cardiac Ischemia Time-Insensitive Predictive Instrument (ACI-TIPI ≥ 75%) or Thrombolytic Predictive Instrument (TPI) Trigger Detecting Presence of ST Elevation Myocardial Infarction (STEMI) (N=437)*
| ACI-TIPI ≥75% or TPI Triggered | ACI-TIPI ≤ 75% and TPI Did Not Trigger | p-value † | |
|---|---|---|---|
| All patients with chest pain or symptoms suggesting ACS | |||
| Proportion with ACS (True positives) | 79.6% (226/284) | 45.2% (66/146) | <0.0001 |
| Proportion not having ACS (False positives) | 20.4% (58/284) | 54.8% (80/146) | |
| Patients who had ACS | |||
| Proportion with AMI | 85.8% (194/226) | 53.0% (35/66) | <0.0001 |
| Proportion with STEMI | 73.0% (165/226) | 25.8% (17/66) | <0.0001 |
Data needed to determine if either ACI-TIPI ≥ 75% or TPI triggered were missing for seven subjects.
Percentages of confirmed diagnoses compared using a chi-square test.
DISCUSSION
When EMS is called to evaluate a patient for chest pain in the community, paramedics must rapidly and accurately identify ACS to initiate appropriate on-site treatment. Moreover, for STEMI, they must match the transport destination hospital with the patient’s potential need for coronary reperfusion. For the IMMEDIATE Trial to test paramedic-administered acute GIK treatment for ACS, the false positive diagnosis rate for ACS among those treated could not exceed 25%. This level of performance also is needed for the effective field implementation of EMS treatment, transport, and communication interventions for ACS, AMI, and STEMI. Yet this is a challenge; this level of performance represents paramedics in the field achieving a false positive rate substantially better than the 50% typical of ED physicians.13
Electrocardiographs with built-in ACI-TIPI and TPI decision support have been shown to improve ED physician diagnosis and treatment of ACS and STEMI, but they had not previously been prospectively studied with paramedics in out-of-hospital settings. In testing, the use of an ACI-TIPI cutoff probability of ACS of ≥ 75% along with the TPI in a wide variety of EMS systems, this study demonstrated that this approach can achieve the needed level of paramedic diagnostic performance for ACS and STEMI.
This investigation also showed that the way ACI-TIPI is used by ED physicians to supplement their clinical judgment, as a continuous 0–100% probability range for ACS, is not optimal for the EMS setting. When in Phase 1, following extensive training in ECG interpretation, ACI-TIPI, and TPI, paramedics used the 0–100% approach, their performance was similar to that seen with ED physicians: 54.2% of their diagnoses for ACS were false positives. While acceptable for the ED diagnosis of ACS/AMI, this is not sufficient for use of treatments directed at ACS and STEMI in the field. Moreover, using the 0–100% continuous ACI-TIPI scale, among patients identified by paramedics as having ACS, only 59.2% had AMI and 40.8% had STEMI, not sufficiently high proportions of AMI and STEMI to justify EMS activating receiving hospital PCI laboratory teams. In contrast, when the ACI-TIPI cutoff and TPI were used, these percentages increased to 85.8% and 73.0%, respectively, considered by the participating hospitals as sufficient for “STEMI Alerts.”
With the 75% ACI-TIPI cutoff/TPI approach tested in Phase 2, the proportion of false positives for ACS dropped to less than half, to 20.4%. Moreover, among those with ACS, in Phase 2, a greater proportion of those identified as having ACS had AMI, 84.8%, and of those with AMI, more had STEMI, 80.6% (68.4% of all those with ACS). This identification of ACS, with a high proportion having AMI and STEMI, met the target of the IMMEDIATE Trial, and would likely meet the needs for a large number of ACS-targeted treatments. From the EMS systems’ perspectives, this met the requirements for EMS identification in the community for treatment and transport of patients with ACS. Moreover, participating EMS systems found the cutoff use of ACI-TIPI with TPI easy to apply in the community settings in which paramedics must evaluate patients, and it required purchase of no additional equipment. (To not disrupt local EMS practices for STEMI identification, including medical control physician oversight, if in use, those patients who met local EMS “STEMI Alert” criteria also were considered as designated by paramedics as having ACS/STEMI and suitable for IMMEDIATE Trial enrollment and treatment.) Used in the wide range of the 11 communities’ EMS systems, and the approach had the same impact.
In this first application of this approach, paramedics did not completely adhere to the ACI-TIPI cutoff and TPI for designation of ACS. Especially at first, in Phase 2 as they changed from the use of the continuous ACI-TIPI score, they still included patients not meeting these criteria. (In Phase 3, when training only included the cutoff use of ACI-TIPI, inclusion was more selective.) Thus, to understand the potential of this approach, we retrospectively classified all patients as if the ACI-TIPI/TPI cutoff approach had been strictly applied. Therefore, the proportion of false positives for ACS was even lower than seen in use in the field; 19.2%, and of patients identified as having ACS, 86.4% had AMI, of whom 84.3% had STEMI identified (72.8% of ACS was STEMI). This suggests that although excellent performance was achieved by just introducing to paramedics the use of the cutoff approach with ACI-TIPI/TPI-capable electrocardiographs, it is possible that with feedback-based improvement14 yet better performance could be achieved.
Of note, the approach used in this study’s diverse EMS systems required no special ECG transmission equipment, systems, or staffing, caused no interference or delay in care, and required no special training to identify STEMI. Indeed, as illustrated in Phase 3, training took only 1.5 hours. Deployment required only having standard ACI-TIPI and TPI software on the EMS electrocardiographs. This approach could provide an attractive option for communities desiring to improve EMS diagnosis of ACS and STEMI while minimizing disruption and cost. This also could help EMS systems identify patients who should be transported directly to PCI-capable centers, and also simultaneously identify patients for whom such special transport for reperfusion is not warranted. This could facilitate appropriate preparation at receiving hospitals, and could help a community use its PCI-capable and community hospitals most effectively.
Currently, half of patients arriving at the ED with symptoms suggestive of ACS come by EMS, and hopefully in the future, more. For these patients, emergency care starts before ED arrival, and better recognition and treatment of ACS prehospital should lead to better outcomes once at the hospital. Especially for seriously ill patients, the hand-off and transition from EMS to ED and hospital care should be enhanced by having shared information and decision support, especially for the time-sensitive treatments for ACS and STEMI. Moreover, with better prehospital recognition of ACS and STEMI, personnel and facilities at the receiving ED can be readied, reducing delays to treatment, including PCI. STEMI Alert15 and Cardiac Point of Entry programs16–18 including EMS and community hospitals in collaborative quality improvement programs have the opportunity to affect the outcomes of STEMI care.19 The approach used in this study should support these programs in realizing their potential.
There are several potential limitations to this study’s results. First, this trial used a before-after quasi-experimental design, not a randomized approach. This was necessary because of the nature of the experimental intervention and practical operations of EMS systems. Once electrocardiographs were programmed to provide ACI-TIPI and TPI results for each patient, and paramedics were trained to use the ACI-TIPI cutoff, it was not possible to expect them to randomly use or not use the 75% cutoff. There was no practical other way we could compare use of the cutoff approach to the continuous-scale approach used in EDs; the most suitable study design was to get baseline performance measures using the usual ACI-TIPI approach before the new approach, and then to institute the intervention. This also has the advantage of having the same EMS systems use both approaches to allow direct assessment of the impact of the intervention. That the impact of the intervention was seen across all sites in Phases 1 and 2, and also when tested in yet more sites in Phase 3, supports the conclusion that the experimental intervention was the cause for the observed change, and had a reproducible impact.
A potential limitation is related to the fact that the prevalence of ACS and general features of a population can vary by setting, which could limit generalizability. In this study, the positive findings across the study’s range of towns’ and cities’ EMS systems, including urban, suburban, and rural, provide evidence for the approach’s wide applicability. A related potential limitation to generalizability in studies of EMS (and ED) triage and treatment can result from limitations of inclusion criteria. For example, if only patients with chest pain are included, the results may not apply to the 13–25% of patients with ACS/AMI who do not have chest pain. Because the IMMEDIATE Trial considers for inclusion all patients with symptoms suggestive of ACS, these results should apply to community-based EMS patients with a range of symptoms suggestive of ACS.
Another challenge of such studies related to the inclusion of all ACS, rather than only AMI or STEMI, is that the diagnosis of ACS is harder to make definitively than the diagnoses of AMI and STEMI. However, identifying unstable angina pectoris as part of ACS is clinically important, not only because it has its own mortality rate, though lower than AMI, but also because monitoring and treatment during the early high-risk period is intended to prevent progression to AMI that may occur in about 9% of unstable angina cases. 20,21 Indeed, the importance of addressing the care of all patients with ACS rather than only AMI and/or STEMI led the NIH National Heart Attack Alert Program to change its initial focus on AMI to all ACS.22 Accordingly, to maximize the clinical relevance of this study in community-based EMS practice, we evaluated our approach for all patients with ACS.
CONCLUSIONS
This study’s results demonstrate that in a wide variety of settings, paramedic use of a cutoff-based ACI-TIPI and TPI in out-of-hospital electrocardiographs can result in excellent identification of ACS, including AMI and STEMI. This approach could facilitate rapid and accurate identification of patients with ACS and AMI/STEMI in communities, and thereby could have an important impact on care and thus clinical outcomes.
Acknowledgments
The author thanks Muriel Powers for expert manuscript preparation.
FUNDING SOURCES
The IMMEDIATE Trial is supported by a grant (U01 HL077821) from the National Heart Lung and Blood Institute.
Footnotes
Clinical Trial Registration Information: www.clinicaltrials.gov, NCT00091507.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Eckstein M, Cooper E, Nguyen T, Pratt FD. Impact of Paramedic Tansport with prehospital 12-lead electrocardiography on door-to-balloon times for patients with ST-segment elevation myocardial infarction. Prehospital Emergency Care. 2009;13(2):203–206. doi: 10.1080/10903120802472020. [DOI] [PubMed] [Google Scholar]
- 2.Lee CH, Van Gelder CM, Cone DC. Early cardiac catheterization laboratory activation by paramedics for patients with ST-segment elevation myocardial infarction on prehospital 12-lead electrocardiograms. Prehospital Emergency Care. 2010;14(2):153–158. doi: 10.3109/10903120903537213. [DOI] [PubMed] [Google Scholar]
- 3.Turnipseed SD, Amsterdam EA, Laurin EG, Lichty LL, Miles PH, Diercks DB. Frequency of non-ST-segment elevation injury patterns on prehospital electrocardiograms. 2010;14(1):1–5. doi: 10.3109/10903120903144924. [DOI] [PubMed] [Google Scholar]
- 4.Davis DP, Graydon C, Stein R, Wilson S, Buesch B, Berthiaume S, Lee DM, Rivas J, Vilke GM, Leahy DR. The positive predictive value of paramedic versus emergency physician interpretation of the prehospital 12-lead electrocardiogram. 2007;11(4):399–402. doi: 10.1080/10903120701536784. [DOI] [PubMed] [Google Scholar]
- 5.Ting HH, Krumholz HM, Bradley EH, Cone DC, Curtis JP, Drew BJ, Field JM, French WJ, Gibler WB, Goff DC, Jacobs AK, Nallamothu BK, O’Connor RE, Schuur JD. Implementation and integration of prehospital ECGs into systems of care for acute coronary syndromes. Circulation. 2008;118:1066–1079. doi: 10.1161/CIRCULATIONAHA.108.190402. [DOI] [PubMed] [Google Scholar]
- 6.Weaver WD, Cerqueira M, Hallstrom AP, Litwin PE, Martin JS, Kudenchuk PJ, Eisenberg M. Prehospital-initiated vs hospital-initiated thrombolytic therapy: the myocardial infarction triage and intervention trial. JAMA. 1993;270:1211–1216. [PubMed] [Google Scholar]
- 7.Aufderheide TP, Michael HK, Hendley GE, Robinson NA, Hastings TE, Lewin RF, Hewes HF, Daniel A, Engle D, Gimbel BK, Bortin KR, Clardy DJ, Schmidt DH, Bajwa T, Holzhauer P, Dabrowski RC, Schuchard GH, Teichman S. Milwaukee Prehospital Chest Pain project – phase I: feasibility and accuracy of prehospital thrombolytic candidate selection. Am J Cardiol. 1992;69:991–996. doi: 10.1016/0002-9149(92)90852-p. [DOI] [PubMed] [Google Scholar]
- 8.Selker HP, Beshansky JR, Griffith JL, Aufderheide TP, Ballin DS, Bernard SA, Crespo SG, Feldman J, Fish SS, Gibler WB, Kiez DA, McNutt RA, Moulton AW, Ornato JP, Podrid PJ, Salem DN, Sayre MR, Woolard RH. Use of the acute cardiac ischemia time-insensitive predictive instrument (ACI-TIPI) to assist emergency department triage of patients with chest pain or other symptoms suggestive of acute cardiac ischemia: a multicenter controlled clinical trial. Ann Intern Med. 1998;129:845–855. doi: 10.7326/0003-4819-129-11_part_1-199812010-00002. [DOI] [PubMed] [Google Scholar]
- 9.Selker HP, Griffith JG, Beshansky JR, Schmid CH, Califf RM, D’Agostino RB, Laks MM, Lee KL, Manyard C, Selvester RH, Wagner GS, Weaver WD. Patient-specific predictions of outcomes in myocardial infarction for real-time emergency use: a thrombolytic predictive instrument. Ann Intern Med. 1997;127:538–556. doi: 10.7326/0003-4819-127-7-199710010-00006. [DOI] [PubMed] [Google Scholar]
- 10.Aufderheide TP, Rowlandson I, Lawrence SW, Kuhn EM, Selker HP. A test of the acute cardiac ischemia time-insensitive predictive instrument (ACI-TIPI) for prehospital use. Ann Emerg Med. 1996;27:193–198. doi: 10.1016/s0196-0644(96)70322-0. [DOI] [PubMed] [Google Scholar]
- 11.Gillum RF, Fortmann SP, rineas RJ, Kottke TE. International diagnostic criteria for acute myocardial infarction and acute stroke. Am Heart J. 1984;108:150158. doi: 10.1016/0002-8703(84)90558-1. [DOI] [PubMed] [Google Scholar]
- 12.Campeau L. Grading of angina pectoris (Letter) Circulation. 1976;54:522–523. [PubMed] [Google Scholar]
- 13.Pope JH, Ruthazer R, Beshansky JR, Griffith JL, Selker HP. Clinical features of emergency department patients presenting with symptoms suggestive of acute cardiac ischemia: a multicenter study. J Thrombosis and Thrombolysis. 1998;6:63–74. doi: 10.1023/A:1008876322599. [DOI] [PubMed] [Google Scholar]
- 14.Daudelin D, Selker HP. Medical Error Prevention in ED Triage for ACS: Use of Cardiac Care Decision Support and Quality Improvement Feedback. Cardiology Clinics. 2005;23:601–614. doi: 10.1016/j.ccl.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Brown JP, Mahmud E, Dunford JV, Ben-Yehuda O. Effect of prehospital 12-lead electrocardiogram on activation of the cardiac catheterization laboratory and door-to-balloon time in ST-segment elevation acute myocardial infarction. Am J Cardiol. 2008;101:158–161. doi: 10.1016/j.amjcard.2007.07.082. [DOI] [PubMed] [Google Scholar]
- 16.Gross BW, Dauterman KW, Moran MG, Kotler TS, Schnugg SJ, Rostykus PS, Ross AM, Weaver WD. An approach to shorten time to infarct artery patency in patients with ST-segment elevation myocardial infarction. Am J Cardiol. 2007;99:1360–1363. doi: 10.1016/j.amjcard.2006.12.058. [DOI] [PubMed] [Google Scholar]
- 17.Morrison LJ, Brooks S, Sawadsky B, McDonald A, Verbeek PR. Prehospital 12-lead electrocardiography impact on acute myocardial infarction treatment times and mortality: a systematic review. Acad Emerg Med. 2006;13:84–89. doi: 10.1197/j.aem.2005.07.042. [DOI] [PubMed] [Google Scholar]
- 18.Moyer P, Feldman J, Levine J, Beshansky J, Selker HP, Barnewolt B, Brown DF, Cardoza JP, Grossman SA, Jacobs A, Kerman BJ, Kimmelstiel C, Larson R, Losordo D, Pearlmutter M, Pozner C, Ramirez A, Rosenfield K, Ryan TJ, Zane RD, Cannon CP. Implications of the mechanical (PCI) vs thrombolytic controversy for ST segment elevation myocardial infarction on the organization of emergency medical services: the Boston EMS experience. Crit Pathways in Cardiol. 2004;3:53–61. doi: 10.1097/01.hpc.0000128714.35330.6d. [DOI] [PubMed] [Google Scholar]
- 19.Boden WE, Eagle K, Granger CB. Reperfusion strategies in acute ST-segment elevation myocardial infarction: A comprehensive review of contemporary management options. J Am Coll Cardiol. 2007;50:917–929. doi: 10.1016/j.jacc.2007.04.084. [DOI] [PubMed] [Google Scholar]
- 20.Unstable angina pectoris: National Cooperative Study Group to Compare Medical and Surgical Therapy. IV. Results in patients with left descending coronary artery disease. Am J Cardiol. 1981 Sep;48(3):517–24. doi: 10.1016/0002-9149(81)90082-5. [DOI] [PubMed] [Google Scholar]
- 21.Krause KR, Hutter AM, Jr, DeSanctis RW. Acute coronary insufficiency. Course and follow-up. Circulation. 1972;45 and 46:S166–S171. [PubMed] [Google Scholar]
- 22.Selker HP, Zalenski RJ, Antman EM, Aufderheide TP, Bernard SA, Bonow RO, Gibler WB, Hagen MD, Johnson P, Lau J, McNutt RA, Ornato J, Schwartz JS, Scott JD, Tunick PA, Weaver ED. An evaluation of technologies for identifying acute cardiac ischemia in the emergency department: A report from a national heart attack alert working group. Ann Emerg Med. 1997;29:13–87. doi: 10.1016/s0196-0644(97)70298-1. [DOI] [PubMed] [Google Scholar]