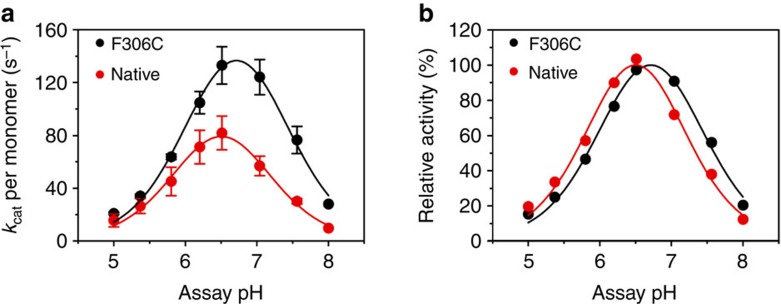
Figure 6. pH dependence of nitrite reductase activity of F306C compared with wild-type AxNiR.
Steady-state activities of NiR activity were measured by using dithionite as electron donor. The oxidation of dithionite was measured spectrophotometrically at 315 nm (?315=8.043 mM−1 cm−1) in a stopped-flow apparatus by using MMH buffer, titrated to the indicated pH, at 4 °C (refs 5, 8). Initial rates were determined from five averaged progress curves and corrected for the background depletion of absorbance at 315 nm in the absence of AxNiR. Data shown are the mean±s.d. of two individual experiments. The activity profile for the wild-type enzyme is different from that published by Abraham et al.34, which was measured by using a stopped-time assay. The optimum for wild-type AxNiR found in the current study is ~pH 6.5, rather than pH 5.2, also no plateau at pH 5.8–6.2 is observed. This ‘new’ optimum pH is similar to the pH optimum of 6.2 found for AcNiR and AfNiR. The pH optimum for F306C is slightly shifted to pH 6.7 compared with the wild-type enzyme. (a) Observed rates versus pH, and (b) relative activity versus pH. The pH dependence of the kinetic parameters were fitted to an equation describing a double ionization35. The calculated macroscopic pKa values are 6.0 and 7.0 for wild-type AxNiR, and 6.1 and 7.3 for F306C.