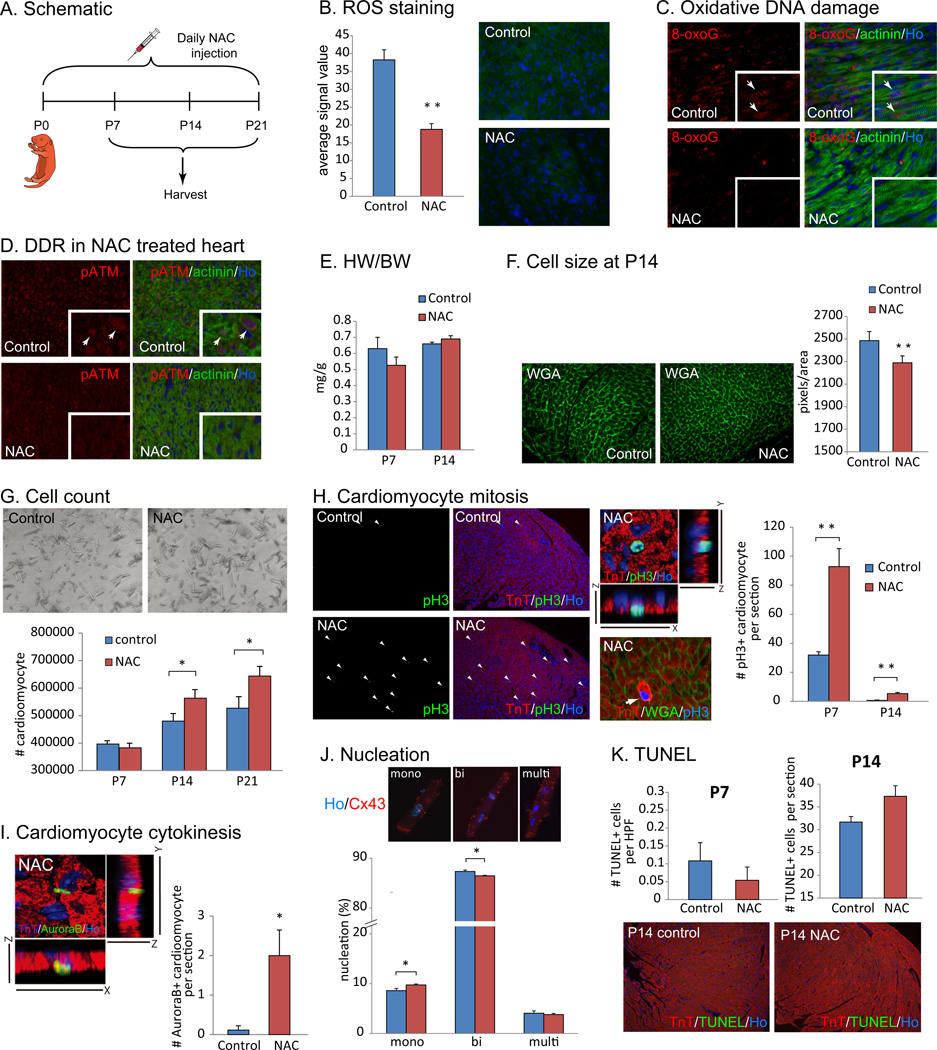
Figure 4.
Injection of a scavenger against ROS suppresses post-natal cardiomyocyte cell cycle arrest. (A) N-acetyl-cysteine (NAC) was administered for 21 days after birth. (B) ROS level in cardiomyocytes significantly decreased as shown by dihydrorhodamine 123 staining in NAC treated neonates. (C) Reduced 8-oxoG staining was seen in NAC injected heart, demonstrating reduced oxidative DNA damage in cardiomyocyte at P7. (D) NAC injection suppressed activation of DNA damage response pathway. Co-immunostaining with anti-pATM and anti-alpha actinin antibodies showed that NAC treatment reduces nuclear phospho-ATM at P7 compared with control (arrows in left panels). (E) HW/BW ratio in control and NAC treated hearts at P14 showed no significant difference. (F) Cell size quantification using WGA staining showed significantly decreased cell size in NAC treated hearts. (G) Total number of cardiomyocyte was significantly increased in NAC treated heart at P21. (H) Co-immunostaining with anti-pH3 and anti-TnT antibodies showed increased cardiomyocyte mitosis in NAC treated hearts. Images shown are hearts from PBS-injected control or NAC-injected neonates at P14. (I) NAC injection induced cardiomyocyte cytokinesis indicated by Aurora B and TnT double positive cardiomyocytes. (J) Percentage of binucleated cardiomyocytes was significantly decreased and mononucleation was siginificantly increased in NAC treated hearts at P14. Immunocytochemistry on isolated myocyte with anti-Cx43 antibody and Hoechst 33258 (Ho) nuclear specific dye were used to visualize boundary and nuclei of each cardiomyocyte, respectively. (K) Apoptotic cell death visualized with TUNEL assay was not increased in NAC-treated heart at neither P7 nor P14. Error bars indicate SEM. *p < 0.05; **p < 0.01.