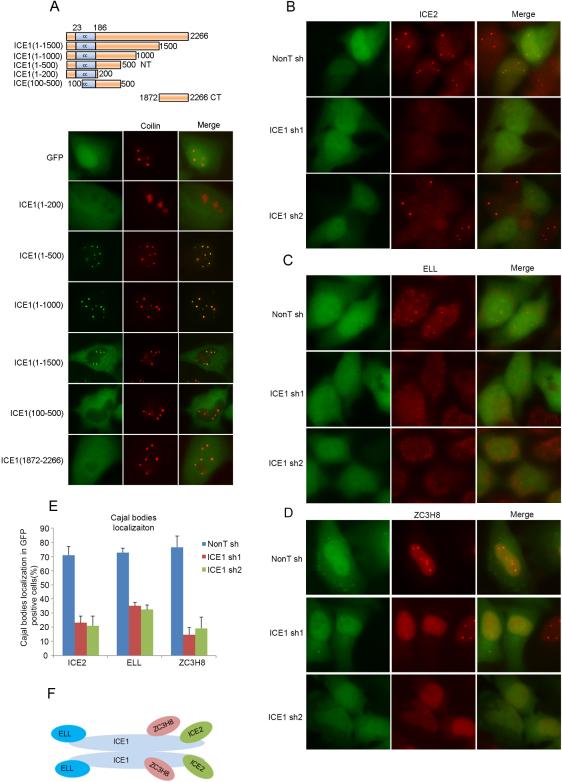
Figure 2. ICE1 is a scaffolding protein for LEC and is required for the targeting of LEC to subnuclear bodies.
(A) The recruitment of ICE1 to coilin-positive subnuclear bodies requires its N-Terminal 500 amino acids, which includes a 163 amino acid coiled-coil domain. Different ICE1 truncations were expressed with an N-terminal GFP tag in HeLa cells and the localization of these ICE1 truncations was visualized by fluorescence microscopy. (B-E) Images (B-D) and their quantitation (E) show that HeLa cells depleted of ICE1 by two different shRNA are defective in the localization of ICE2 (B), ELL (C), and ZC3H8 (D) to subnuclear bodies. For (E), more than 100 GFP-positive cells were scored for whether bright nuclear dots of ICE2, ELL, or ZC3H8 were present, indicative of coilin body localization. Error bars represent the standard deviations. (F) Proposed molecular architecture of the LEC complex in human cells. The LEC complex might dimerize through the N-terminus of ICE1 (data not shown). ICE2 and ZC3H8 associate with ICE1 through its C-terminal portion while ELL binds to the N-terminal region (see Figure S2 for related information).