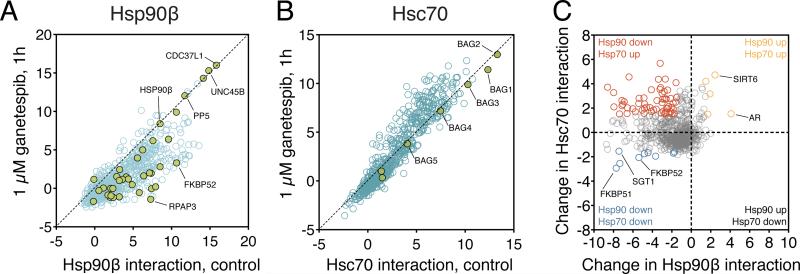
Figure 4. Effect of transient Hsp90 inhibition on chaperone interactions.
A) Hsp90 inhibition leads to dissociation of most clients from Hsp90β. Hsp90β was surveyed for interaction with 800 proteins with LUMIER assay. Cells were treated for 1 hour with 1 µM ganetespib or left untreated before the assay. Hsp90 co-chaperones are shown as green circles. Interaction strength was quantitated as LUMIER scores. B) Hsp90 inhibition leads to stronger association of some proteins with Hsc70. Hsc70 was assayed for interaction with 800 proteins as in (A). Hsp70 cochaperones are shown as green circles.
C) Comparison of the effects of ganetespib on Hsp90β and Hsc70 interactions. The plot shows the change in interaction of 800 tested proteins with Hsp90β and Hsc70 (change is defined as LUMIER score drug – LUMIER score control). Proteins that show differential association with both chaperones are indicated in orange (both increase), blue (both decrease) or red (decrease in Hsp90β interaction, increase in Hsc70 interaction). Selected proteins are labeled.