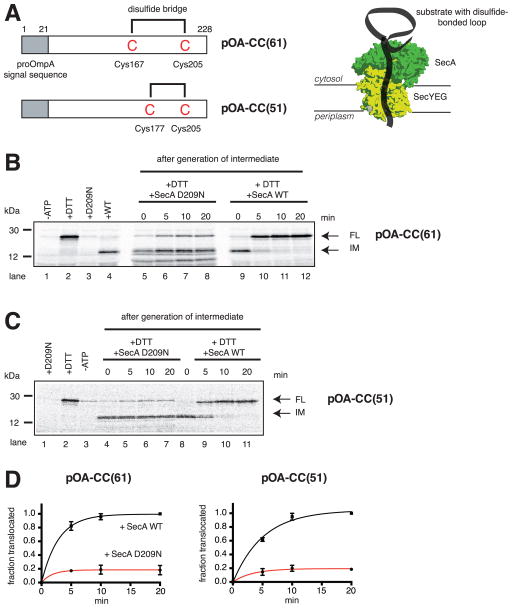
Figure 1. SecA is moderately processive.
(A) Generation of translocation intermediates. The substrates pOA-CC(61) and pOA-CC(51) contain a signal sequence (grey box) and cysteines (C) at the indicated positions, which form a disulfide-bonded loop that prevents complete transport through the complex of SecA and SecYEG (shown in green and yellow, respectively).
(B) A translocation intermediate was generated with pOA-CC(61), wild type (WT) SecA, ATP, and proteoliposomes containing SecYEG. Then, a 64fold molar excess of either the dominant-negative mutant SecA D209N or WT SecA was added together with DTT. Samples were taken at the indicated time points, treated with proteinase K, and analyzed by SDS-PAGE and autoradiography. In lanes 1–4, the indicated components were added or omitted before generation of the intermediate. IM and FL indicate protease-protected fragments, corresponding to the intermediate and fully translocated pOA-CC(61), respectively. Molecular weight markers are indicated (in kDa).
(C) As in (B), but with pOA-CC(51).
(D) Quantification of the experiments in (B) and (C). Plotted is the amount of FL relative to the final levels of FL seen with WT SecA (means and standard deviations of three experiments).