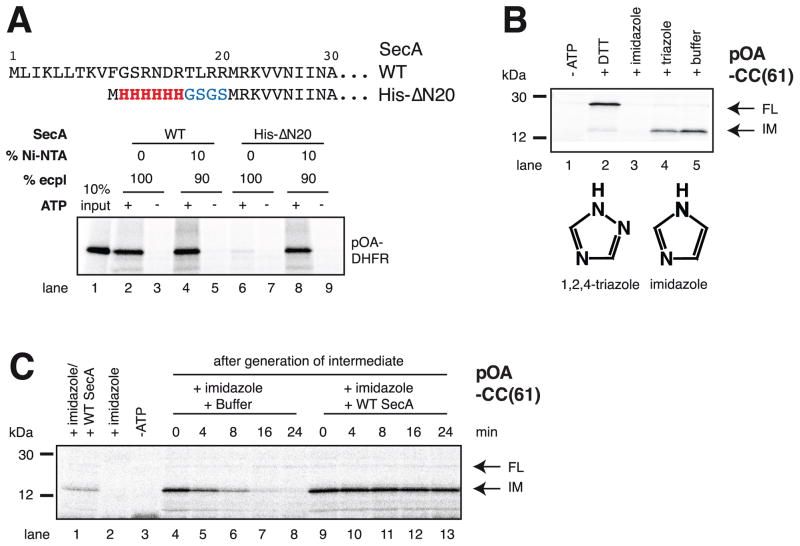
Figure 2. SecA rebinds to the translocating SecY channel through prior interaction with lipids.
(A) N-terminal sequences of wild type (WT) SecA and of the His-ΔN20 mutant. These proteins were incubated with radiolabeled substrate (a fusion of proOmpA and DHFR; pOA-DHFR), ATP, and proteoliposomes containing SecYEG and either 100% E. coli polar lipids (ecpl) or 90% ecpl and 10% Ni-NTA lipids. Translocation of pOA-DHFR was assessed by proteinase K treatment followed by SDS-PAGE and autoradiography. 10% of the input material was loaded in lane 1.
(B) SecA His-ΔN20 was incubated with radiolabeled pOA-CC(61), ATP, and Ni-NTA proteoliposomes containing SecYEG. Hexokinase/glucose (-ATP), DTT, imidazole, or triazole were added, as indicated, before the start of the translocation reaction. The samples were analyzed as in (A). IM and FL indicate protease-protected fragments, corresponding to the intermediate and fully translocated pOA-CC(61), respectively. Molecular weight markers are indicated (in kDa). The lower panel shows the structures of imidazole and triazole.
(C) As in (B), but imidazole was added after generation of the translocation intermediate, together with buffer or WT SecA. In lanes 1–3, the indicated components were added before generation of the intermediate.