Fig.4. Phosphorylation of Wee1B by CaMKII.
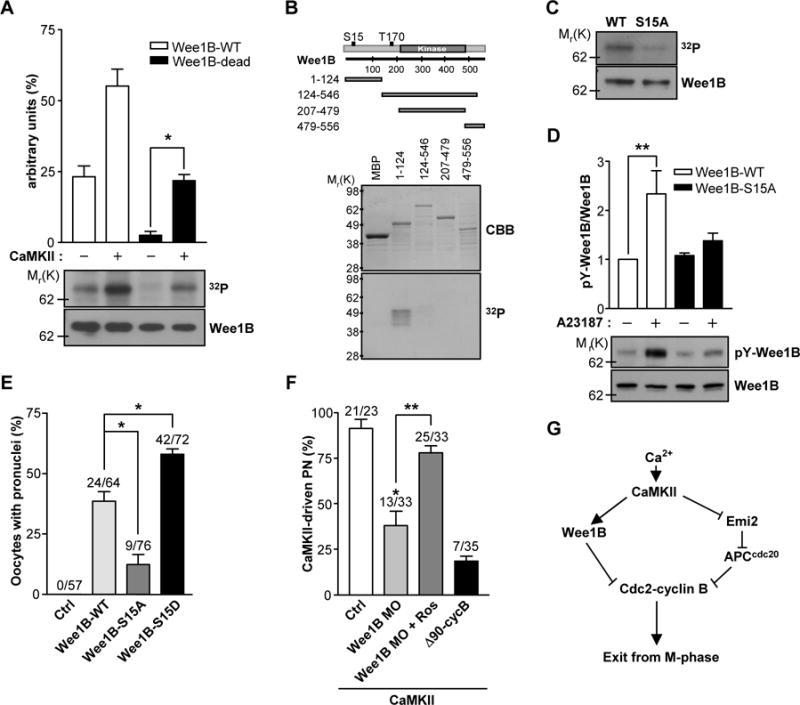
(A) Wee1B was overexpressed in HEK293 cells, immunoprecipitated, and incubated with or without CaMKII in the presence of [γ-32P]ATP for 10 min. The phosphorylation state was detected by SDS-PAGE and autoradiography. The amounts of loaded protein were determined by immunoblot. A representative experiment of the three performed is shown. (B) MBP-tagged truncated forms of Wee1B were generated and expressed in Escherichia coli. After purification by affinity chromatography, fusion proteins were incubated with CaMKII in the presence of [γ-32P]ATP for 10 min. The radiolabeled fusion proteins were detected by autoradiogram. The expression and purification of MBP fusion proteins were monitored by Coomassie brilliant blue (CBB) staining. (C) Immunoprecipitated Wee1B-WT or -S15A mutant were incubated with CaMKII in the presence of [γ-32P]ATP for 10 min, and incorporation of 32P was detected by autoradiography. The amounts of loaded protein were determined by immunoblot. (D) HEK293 cells overexpressing Wee1B-WT or -S15A mutant were treated with 10μM of A23187 for 20 min. The activity of Wee1B was measured by the autophosphorylation of tyrosine residues. A representative image of three independent experiments performed is reported. (E) MII oocytes were injected with mRNAs encoding the indicated Wee1Bs, and oocytes with a pronucleus were counted after 6 hours. (F) MII oocytes injected with Wee1B MO or Δ90cyclin B mRNA were microinjected with constitutively active CaMKII (CA-CaMKII) and pronuclear formation scored after 8 hours. *p<0.005 and **p<0.05 (G) Proposed model of mouse egg activation.
