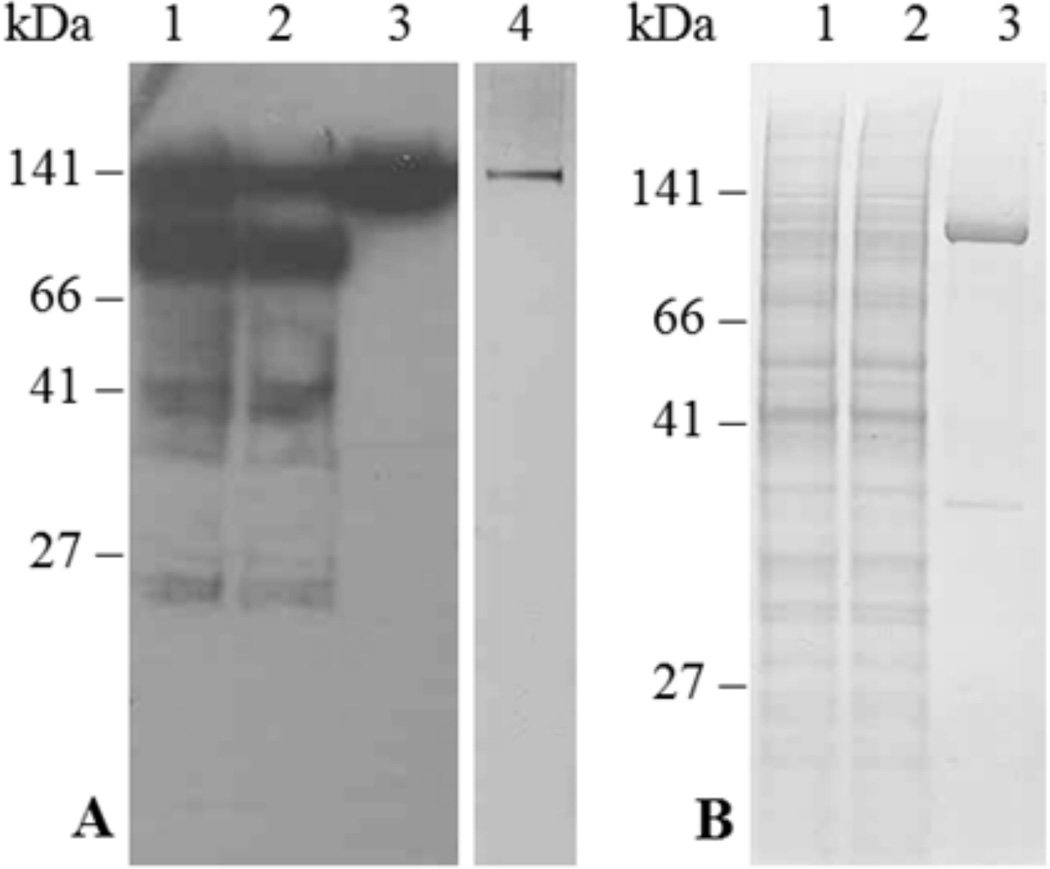
Fig. 5.
Purification of ACA12-GFP (panel A) and ACA12 (panel B) by CaM-Sepharose affinity. Yeast microsomal proteins (100 mg), solubilized with n-dodecyl β-d-maltoside, were incubated overnight with CaM-Sepharose; after collection of the unbound protein fraction, the resin was washed as described in the Materials and Methods and CaM-bound proteins were eluted with 4 ml of elution buffer containing 2 mM EGTA. Panel A: lane 1, 10 µl of solubilized microsomal proteins (40 µg); lane 2, 10 µl of the unbound protein fraction; lane 3, 9 µl of the EGTA-eluted fraction; lane 4, 1 µl of the EGTA-eluted fraction concentrated ten-fold. Fractions were subjected to SDS-PAGE, blotted and decorated with the HisProbe-HPR (lanes 1–3) or subjected to SDS-PAGE and silver-stained (lane 5). Panel B: lane 1, 10 µl of solubilized microsomal proteins (40 µg); lane 2, 10 µl of the unbound protein fraction; lane 3, 3.5 µl of the EGTA-eluted fraction concentrated twenty-fold. Fractions were subjected to SDS-PAGE and stained with Comassie blue.