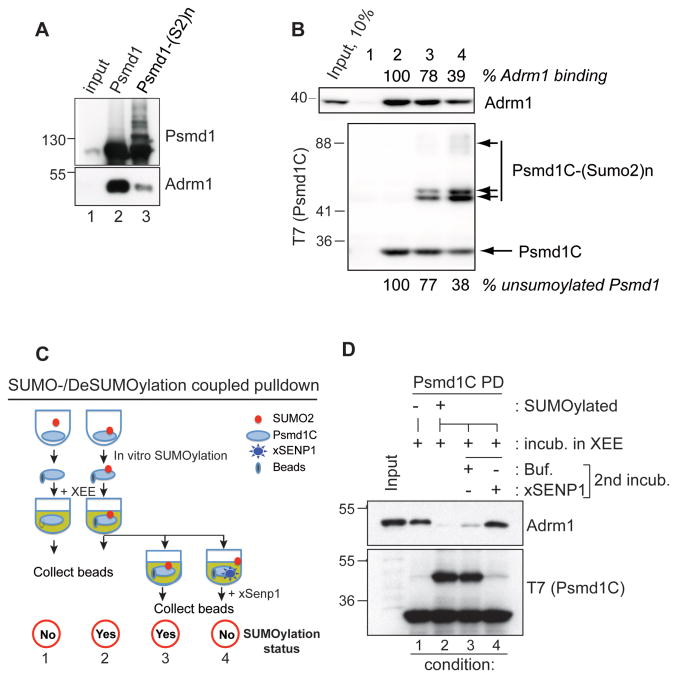
Figure 3. SUMOylation of the Psmd1 C-terminus negatively regulates its interaction with Adrm1.
(A) Full length MBP-Psmd1 was either mock-treated (lane 2) or subjected to in vitro SUMOylation (lane 3), followed by incubation with XEE. The samples were subjected to affinity chromatography, followed by Western blotting of the bound fractions with anti-Psmd1 (upper panel; bracket indicates SUMOylated Psmd1) or anti-Adrm1 (lower panel). Input: 5% of mock-treated input reaction.
(B) A T7-tagged C-terminal fragment of Psmd1 (Psmd1C) was subjected to in vitro SUMOylation for 0 (lane 2), 20 (lane 3) and 60 min (lane 4). The beads were incubated in CSF-XEE, re-isolated and washed. Bound proteins were analyzed by Western blotting with anti-Adrm1 (upper panel) or anti-T7 (lower panel). Lane 1 shows a control sample with empty beads. The amounts unSUMOylated T7-Psmd1C (below lower panel) and of Adrm1 bound to the beads (above upper panel) were quantitated for reactions containing T7-Psmd1C, and normalized relative to levels in lane 2. Input: 10%
(C) Schematic of experiment Figure 3D. In vitro SUMOylation reactions of T7-Psmd1C containing one volume (1x) or three volumes (3x) were incubated without or with ATP, respectively, followed by proportional addition of CSF-XEE and further incubation for 30 min at 23°C. T7-Psmd1C-bound proteins were isolated from the first reaction (-ATP) on beads. The latter (3x) was split into three equal portions; T7-Psmd1C-bound proteins were isolated on beads from the first portion without further manipulation. The second and third portions were supplemented with buffer or xSENP1 catalytic domain, respectively, and incubated for 30 min at 23°C, followed by capture of T7-Psmd1C-bound proteins on beads. All samples were eluted with 1x sample buffer.
(D) Proteins prepared as in (C) were analyzed by Western blotting with anti-Adrm1 and anti-T7. Buf. = buffer. Input: 5%