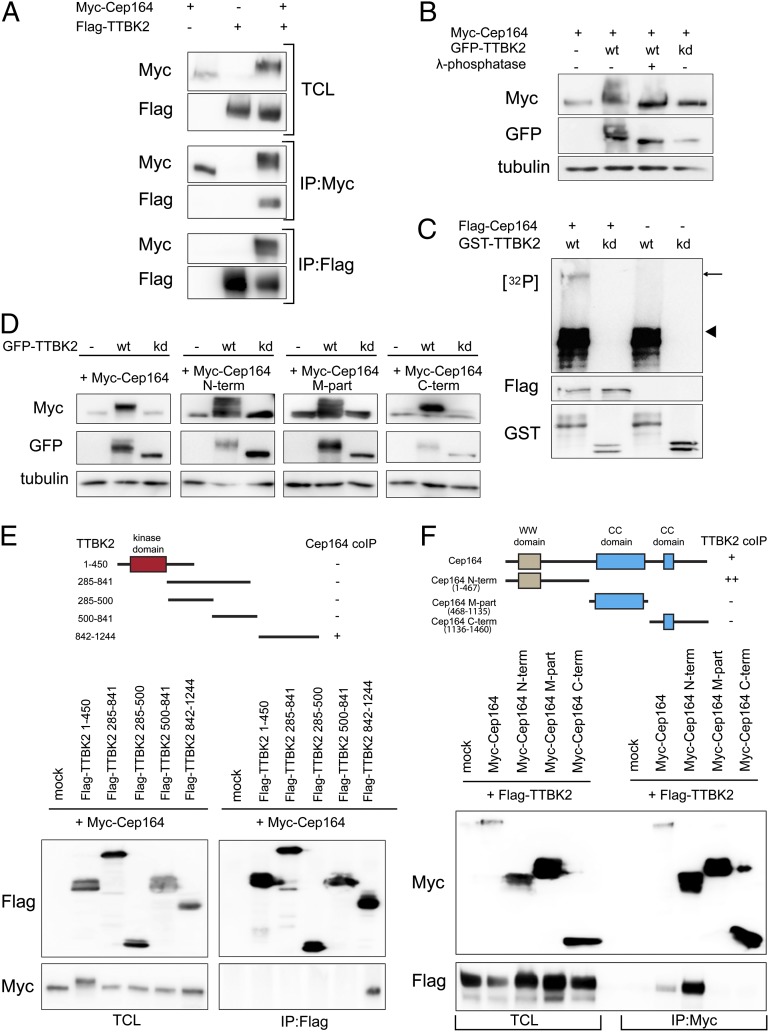
Fig. 3.
Cep164 is a binding partner and substrate of TTBK2. (A) HEK293T cells were transfected with the indicated constructs and subjected to immunoprecipitation (IP)–Western experiments. Upper shows total cell lysate (TCL). Western blots were performed by using the anti-tag antibodies listed to the left, and IPs were performed by using either anti-Myc (Myc–Cep164; Middle) or anti-Flag antibodies (Flag–TTBK2; Bottom). (B) Protein extracts, prepared from HEK293T cells transfected with Myc–Cep164 and GFP–TTBK [wild-type (WT) or kinase dead (kd) mutant], were treated with or without λ-phosphatase and analyzed by Western blotting. Note that Cep164 undergoes TTBK2-mediated phosphorylation, as indicated by a mobility shift that is sensitive to λ-phosphatase treatment. (C) In vitro-translated Flag–Cep164 was immunoprecipitated by using anti-Flag antibody and subjected to a kinase assay in the presence of [γ-32P]ATP and recombinant GST–TTBK2 (1-450) WT or kd protein. Arrow and arrowhead point to phosphorylated Flag–Cep164 and autophosphorylated GST–TTBK2 (1–450), respectively. (D) Protein extracts of transfected HEK293T cells were analyzed by Western blotting. Note that expression of GFP–TTBK2, but not kd mutant, led to prominently retarded electrophoretic mobility of Myc-tagged Cep164, Cep164 N term, Cep164 M part, and Cep164 C term. Also, note concomitant increases in levels of Cep164 and Cep164 C term. (E and F) Transfected HEK293T cells were subjected to IP–Western experiments. (Upper) Schematic representations of the constructs used are shown, and the symbols +, ++, or − summarize the efficacy of coimmunoprecipitation for each combination. (E) Domain mapping of TTBK2 identifies the C-terminal part (842–1244) as sufficient for Cep164 binding. (F) Domain mapping of Cep164 identifies the N-terminal part (1–467) as sufficient for interaction with TTBK2.