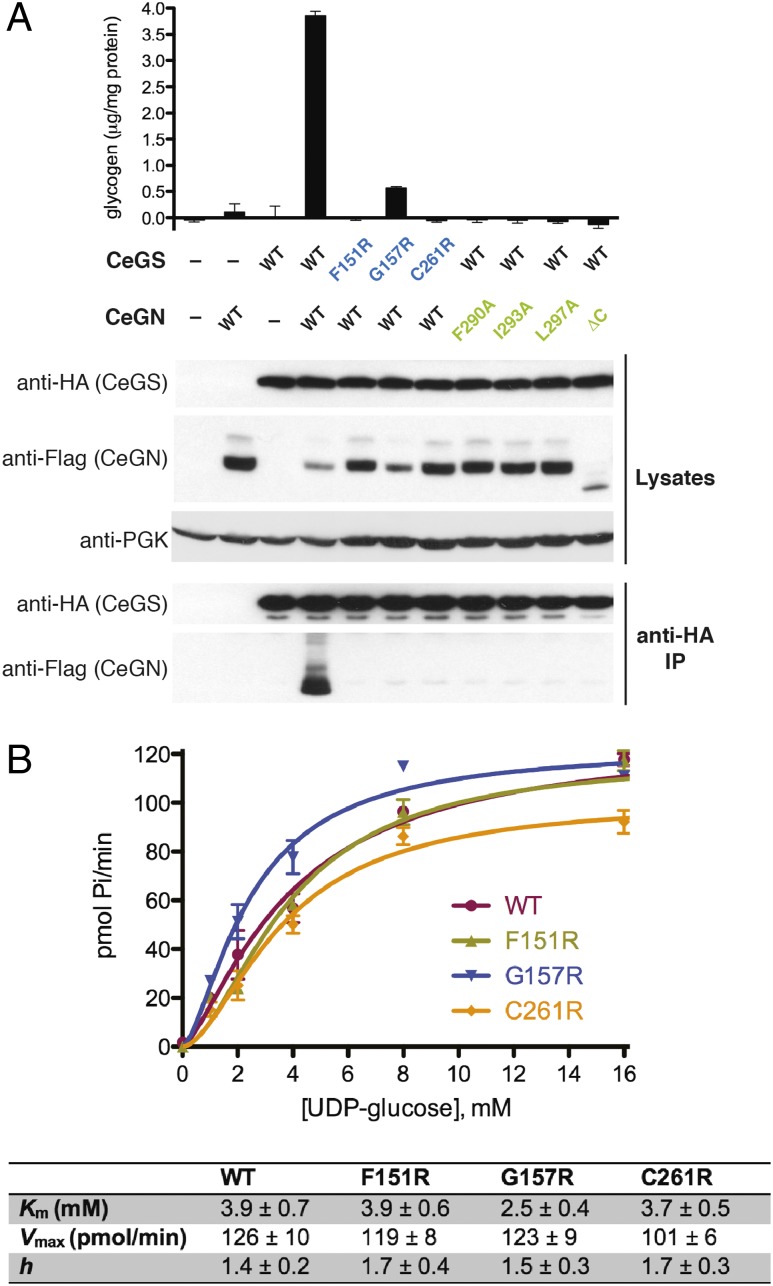
Fig. 4.
The CeGS–CeGN34 interaction is required for glycogen formation in yeast. (A) The indicated versions of CeGS and CeGN were expressed in a gsy1Δ gsy2Δ glg1Δ glg2Δ quadruple deletion yeast strain that lacked endogenous GS and GN. After cell lysis, samples were analyzed for glycogen accumulation (Upper) and for GS–GN interaction by anti-HA immunoprecipitation and either an-HA or anti-FLAG immunoblot (Lower). Anti-PGK (phosphoglycerate kinase) signal was used as a loading control. (B) Glucosylation activity of wild-type and mutant forms of GS expressed and purified from the gsy1Δ gsy2Δ glg1Δ glg2Δ yeast strain. The ability of GS to extend maltooctaose chains in the presence of GBE, 3 mM G6P and increasing UDP-G concentrations was assessed by monitoring phosphate (Pi) release in a coupled colorimetric assay. Kinetic parameters for UDP-G ± SD are the average of two independent experiments.