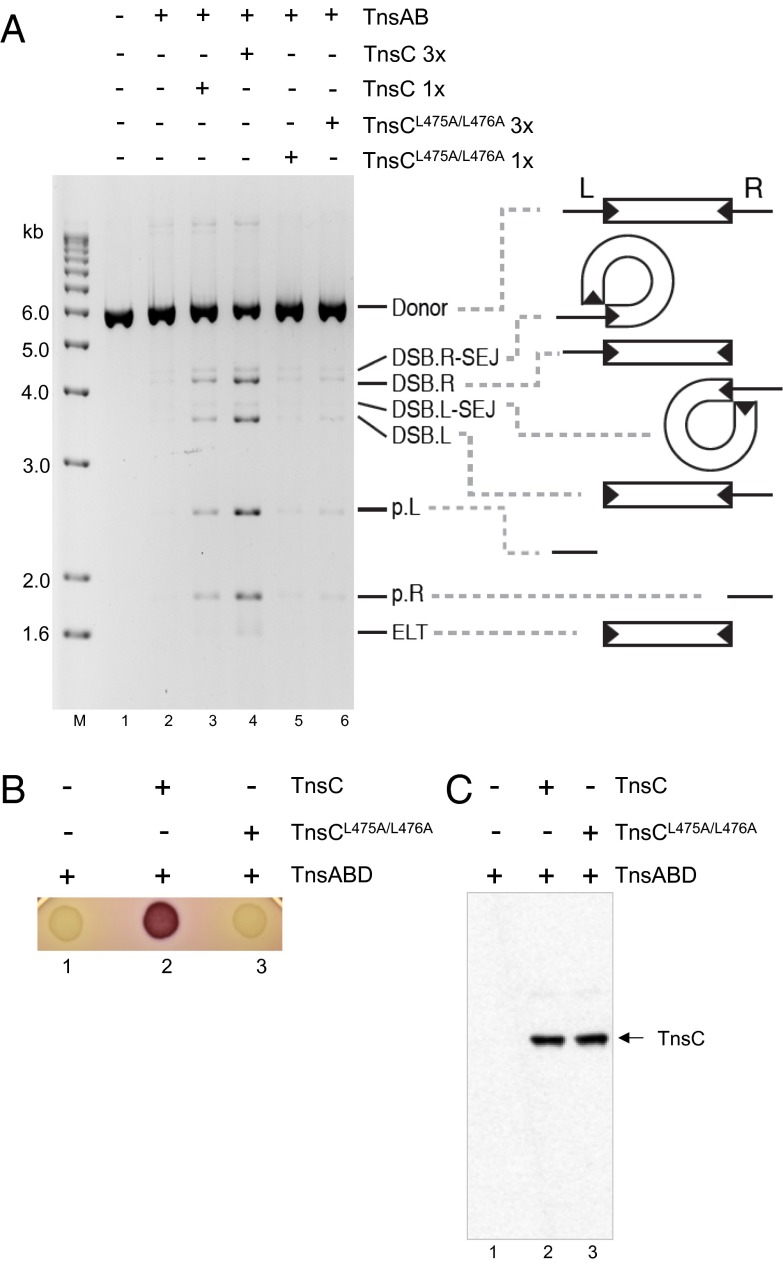
Fig. 3.
The TnsC mutant TnsCL475A/L476A is not active in transposition in vitro or in vivo. (A) TnsCL475A/L476A does not stimulate TnsAB-dependent intramolecular breakage and joining in vitro. In intramolecular breakage and joining reactions, DSBs occur at either or both Tn7 ends to form DSB.L, DSB.R, and ELT species along with plasmid backbone fragments p.L and p.R. Intramolecular joining of the newly exposed Tn7 end 3′OH to its 5′ end results in an DSB.L.SEJ and a DSB.R-SEJ DSBs. A Tn7-containing plasmid was incubated with TnsAB and TnsC derivatives as indicated in the presence of high glycerol concentrations (20% vol/vol), digested with a restriction enzyme that cleaves once in the donor backbone, and displayed on an agarose gel. Lane 1, DNA without protein additions; lane 2, Tn7 plasmid incubated with TnsAB in the absence of TnsC; lanes 3 and 4, Tn7 plasmid incubated with TnsAB and 1× and 3× WT TnsC as indicated; lanes 5 and 6, Tn7 plasmid incubated with TnsAB and 1× and 3× mutant TnsCL475A/L476A as indicated. (B) The TnsC mutant TnsCL475A/L476A is not active in transposition in vivo. Tn7 transposition in vivo was assayed by identification of Lac+ (red) cells using MacConkey agar in a strain containing a miniTn7::lac element that lacks an internal promoter and is located on an F plasmid such that lac is not expressed from an external promoter. Transposition proteins TnsA, TnsB, and TnsD were supplied from a pACYC plasmid, and WT and TnsC TnsCL475A/L476A were supplied from a pUC-based plasmid. TnsABC+D transposition results in translocation of the miniTn7::lac to attTn7, where rare opposite-orientation insertions position the lac genes downstream of the glmS to yield Lac+ (red) cells. Lane 1, TnsABD + vector; lane 2, TnsABD + TnsC; lane 3, TnsABD + TnsCL475A/L476A. (C) TnsC levels in the indicated cultures were measured by Western blot analysis with TnsC antibody.