Abstract
Glutamate receptor activation-mediated excitotoxicity has been hypothesized to cause cell death in both acute and chronic neurodegenerative diseases including glaucoma. Although the precise mechanisms of ischemia-induced neuronal death are unknown, glutamate excitotoxicty-induced apoptotic cell death is considered to be an important component of postischemic damage in the retina. The blockade of apoptotic cell death induced by glutamate receptor activation provides strong evidence that glutamate excitotoxicity-induced apoptotic cell death may be a central mechanism of cell death in ischemic rat retina. We have shown that there is TUNEL-positive apoptotic cell death in the outer nuclear layer, inner nuclear layer and ganglion cell layer of the ischemic rat retina at 12 h.
Keywords: retina, ischemia, glutamate receptor, excitotoxicity, MK801, apoptotic cell death, TUNEL
1. Introduction
Ischemia-induced damage of the retina is a relatively frequent event occurring in the course of a variety of pathological processes. This and the accessibility of the retina for manipulation of blood flow have promoted the use of different experimental models to investigate neuronal responses following ischemia-reperfusion injury (1-8). Since the retina is organized in discrete cell layers, this model also facilitates to study the effects of transient ischemia on different types of neurons. As in other central nervous system tissues, retinal ischemia results in apoptotic neuronal cell death. Most studies on the postischemic retina have reported that cell death is pronounced in the inner retina, which comprises the innermost ganglion cell layer (GCL) and the inner nuclear layer (INL) containing the somata of amacrine, bipolar and horizontal cells, and those of the retina-specific Muller glia (6, 9-11). Although the precise mechanisms of ischemia-induced neuronal cell death are unknown, excitotoxicity triggered by the overactivation of glutamate receptors is considered to be a central component of postischemic damage in the retina (1, 12, 13). The preferential susceptibility of inner retinal neurons to ischemic damage would be in line with this concept, since expression of glutamate receptors is confined to neurons of the GCL and INL (14, 15).
Apoptosis is the most common form of eukaryotic cell death that can be classified by its morphological, biochemical, molecular and functional aspects (16, 17). It has been reported that apoptosis is accompanied by rounding-up of the cell, retraction of pseudopdes, reduction of cellular volume (pyknosis), chromatin condensation, nuclear fragmentation (karyorrhexis), classically little or no ultrastructural modifications of cytoplasmic organelles, plasma membrane blebbing and engulfment by resident phagocytes, in vivo (18). Commercial availability of enzymatic in situ labeling of DNA strand breaks using Terminal deoxynucleotidyl transferase (TdT), which catalyzes polymerization of labeled nucleotides to free 3′-OH DNA ends in a template-independent manner (TUNEL-reaction), and fluorescein labels provides a precise, fast and simple, non-radioactive technique to detect and quantify apoptotic cell death at single cell level in cells and tissues.
2. Materials
2.1. Transient Retinal Ischemia
3-month-old female Sprague-Dawley rat (200-250 g, Harlan Laboratories, Indianapolis, IN). (See Note 1).
Ketamine (100 mg/kg, Ketaset; Fort Dodge Animal Health, Fort Dodge, IA). Store at room temperature.
Xylazine (9 mg/kg, TranquilVed, St. Joseph, MO). Store at room temperature.
Sodium chloride is dissolved in sterilized water at 0.9%. Store at 4°C.
MK801 (Sigma). Working solution (10 mg/kg) is prepared by dilution in 0.9% saline. Store at 4°C.
2.2. Tissue Preparation
2.2.1. Fixation
Paraformaldehyde working solution (4%). 4 g Paraformaldehyde (Sigma) is dissolved in 60 ml of warmed (40-50°C), distilled water, add 2 drops of 0.1N NaOH, and adjust up to 100 ml with 5X PBS. Filter with #1 Whatmann paper. The working solution should be prepared freshly in each experiment.
0.1N Sodium hydroxide. Store at room temperature.
Ice-chilled 5X and 1X phosphate buffered saline (PBS; Fisher Scientific). Store at 4°C.
30-gauge needle and perfusion motor (L/S Analog Console Precision Pump Systems, Barnant Company, Barrington, IL).
Scissors, forceps and 100 mm dish (Fisher Scientific).
#1 Whatmann filter paper (GE Healthcare Bio-Sciences, Piscataway, NJ).
2.2.2. Wax Embedding
Ethanol (Sigma) is diluted with distilled water (50, 70, 80, 90, and 95%). Store at room temperature.
Cetyl alcohol (Sigma). Store at 4°C. (See Note 2).
500g Poly (ethylene glycol) (400) distearate (PEGD;, Polysciences, Inc., Warrington, PA) is melted at 57°C and then mixed with 55.5 g of cetyl alcohol with stirring on hot plates at 60°C. Prepared pure wax can be labeled with pure wax I and II. Store at 4°C. (See Note 3).
Intermediate wax solution is mixed with ratio 1:1 (100% Ethanol : Pure wax). (See Note 3).
Forceps.
20 mL Disposable scintillation vials.
Cryomold Biopsy, Disposable Vinyl Specimen Molds (10 mm × 10 mm × 5 mm) (Tissue-Tek, Torrance, CA).
2.2.3. Gelatin Coating
4.11g Gelatin from porcine skin (Type A; Sigma) is dissolved in heated distilled water (800 ml, final 0.05%, 40-50°C) with stirring. Working solution should be freshly prepared for each slide coating. When the gelatin is thoroughly dissolved, chromium (III) potassium sulfate dodecahydrate (400 mg) is added with stirring. Once the gelatin working solution is thoroughly dissolved, the working solution is filtered with #1 Whatmann filter paper. Store at room temperature. (See Note 4).
#1 Whatmann filter paper (GE Healthcare Bio-Sciences).
Microscope slides (Superfrost; Fisher Scientific). (See Note 5).
2.3. TUNEL staining
Xylazine and Ethanol (Sigma).
0.1% Sodium citrate solution (Sigma) is prepared by dilution with distilled water. Store at room temperature.
0.15% Triton X-100 solution (Sigma) is prepared by dilution with 0.1% sodium citrate solution. Store at room temperature.
20 mg/ml Proteinase K (Sigma). The stock solution is prepared by dilution with 10 mM Tris/HCl, pH 7.4-8. Store at -20°C. The working solution (20 μg/ml) is prepared by dilution with 1X PBS.
In Situ Cell Death Detection Kit (Flurorescence, Roche Applied Science, Indianapolis, IN). Store at -15 to -25°C. Kit contents: Enzyme Solution (Terminal deoxynucleotidyl transferase from calf thymus, recombinant in E.Coli, in storage buffer, 10X conc.) and Label Solution (Nucleotide mixture in reaction buffer, 1X conc.).
3000 U/mL-3 U/mL DNase I recombinant in 50 mM Tris-HCl, pH 7.5, 1 mg/mL, (Roche Applied Science). Store at -20°C.
1% BSA dissolved in PBS and aliquoted with 1 mL in 1.5 mL eppendorf tube. Stable at -20°C for up to 1 year.
10 mg/ml Hoechst 33342 (Trihydrochloride, trihydrate; Invitrogen, Eugene, OR) for nuclear staining. Working solution is freshly prepared by diluting 1:10000 in PBS Store at 4°C.
Fluoromount-G (Southern Biotech, Birmingham, AL) for antifade mounting. Store at room temperature.
3. Methods
3.1 Transient Retinal Ischemia
Female Sprague-Dawley rats, 3 months of age (200–250 g in weight), are anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (20 mg/kg) by intraperitoneal (IP) injection.
A cannula is inserted into the anterior chamber that was connected by flexible tubing to a reservoir as shown inr. Fig. 1.
By raising the reservoir, intraocular pressure is elevated above systolic blood pressure (100–120 mmHg) for 60 min. Animals are allowed to recover for 3 to 24 h.
Fig. 1.
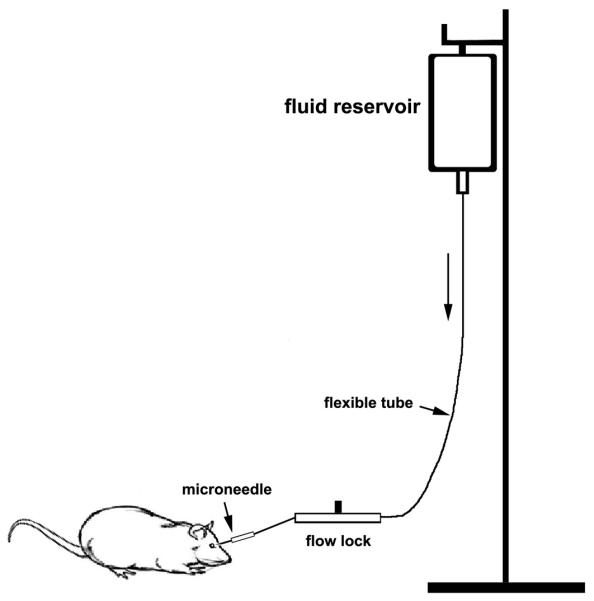
Diagram of the instrument system used to elevate acute intraocular pressure.
3.2 Tissue Preparation
The light-adapted rats are anesthetized with isoflurane followed by an IP injection of a mixture of ketamine/xylazine as above.
Both eyes are enucleated and then the rats were killed by CO2 inhalation.
The retinas are dissected from the choroid and fixed in 5 mL of 4% paraformaldehyde in 1X PBS (pH 7.4) for 4 h at 4°C.
After 1X PBS rinse, retinas are dehydrated through 5 mL of graded ethanols (50, 70, 80, 90, 95, and 100%) at room temperature, immersed intermediate wax solution, pure wax I solution., and pure wax II solution. at 37°C. The retinal tissues are then embedded in pure wax and placed at room temperature. The wax blocks for retinal tissues are stored at 4°C.
The wax blocks for retinal tissues are cut by 5 to 7 μm-thickness using microtome (Reichert-Jung 2030, McBain Instruments, Chatsworth, CA).
The retinal sections are attached onto gelatin-coated glass slides using the water floating method. (See Note 6).
The slides that have retinal tissues are stored at 4°C until use.
3.3 TUNEL Staining
The retinal tissue sections are dewaxed with xylene, and rehydrated with a graded series of ethanol (100, 95, 90, 80, and 70%) and distilled water. Each step is processed for 5 min at room temperature.
After 1X PBS rinse for 10 min, the sections are incubated with proteinase K working solution (20 μg/mL in PBS) for 7 to 10 min at 37°C. (See Note 7).
The sections are rinsed with 1X PBS three times for 5 min.
Prepare a TUNEL reaction mixture with 10 μL of Enzyme solution (vial 1) and 490 μL of Label solution (vial 2) and mix well to equilibrate components at room temperature. (See Note 8).
Each section is incubated with 50 μL of a TUNEL reaction mixture in a humidified chamber for 60 min at 37°C. (See Note 9).
The sections are rinsed with 1X PBS three times for 5 min.
The sections are incubated with 1 μg/mL Hoechst 33342 in 1X PBS for 10 min at room temperature to stain the nuclei.
The sections are rinsed with 1X PBS three times for 5 min.
The sections are mounted with Fluoromount-G and stored at 4°C until imaging.
The images are captured under fluorescence microscopy (Eclipse microscope, model E800; Nikon Instruments Inc., Melville, NY) equipped with a digital camera (SPOT; Diagnostic Instrument, Sterling Heights, MI). Excitation at 494 nm induces the Fluorescein (green emission) for the TUNEL-positive cells, while excitation at 350 nm induces Hoechst 33342 (blue emission). Software is used by Simple PCI version 6.0 software (Compix Inc., Cranberry Township, PA). An example of the signal for TUNEL-positive cells is shown in Fig. 2.
Fig. 2.

Blockade of glutamate receptor activation blocks apoptotic cell death in ischemic rat retina. (A) There were no TUNEL-positive cells in the normal retina. (B) In ischemic retina of rats receiving vehicle, apoptotic cell death was present in the ONL, INL and GCL (arrows). (C) MK801 treatment prior to ischemia blocked apoptotic cell death in all layers. ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Size bar represents 20 μm (A-C). The images were obtained on a Nikon Eclipse fluorescence microscope using a 40X lens as described (1). The sections were counterstained with the nucleic acid stain Hoechst 33342/PBS. (Reproduced from ref. 1 with permission from Molecular Vision.)
Acknowledgements
This work was supported by National Institute Health grant R01 EY018658 (WKJ).
4. Notes
All procedures concerning animals were in accordance with ARVO Statement for the Use of Animals in Ophthalmic Vision Research and under protocols by institutional IACUC committees at the University of California San Diego. The Sprague-Dawley rats were housed in covered cages, fed with a standard rodent diet ad libitum, and kept on a 12-h light/12-h dark cycle.
Cetyl alcohol should be melted in an oven at 60°C overnight for mixing with PEGD.
PEGD (500g) should be melted in an oven at 60°C overnight. Both pure wax I and II, and intermediate wax solutions can be melted at 50°C in the oven overnight for use in an experiment the following day. Both wax solutions can be reused. Stable at 4°C for up to 1 year.
The gelatin should not be added to distilled water that exceeds 50°C because high temperature can denature the gelatin.
Glass slides should be clean before coating. The slides can be placed in gelatin working solution for 2 min, removed from solution, covered by aluminum foil, and dried in refrigerator for 1 h. When slides are completely dried, they can be placed in gelatin coating solution for an additional two times, allowed to dry between each coating, and kept to dry completely in refrigerator for overnight. Stable at 4°C for up to 6 months.
Because the melting point of wax is 37°C, distilled water at room temperature should be used to spread and attach intact tissue sections onto glass slides.
The incubation time for proteinase K is important because proteinase K working solution can induce detachment of retinal tissues from coated glass slides. We have found 7 min is the best incubation time to preserve tissues on glass slides.
The TUNEL reaction mixture should be prepared immediately before use and kept on ice until use. The dilution rate for TUNEL reaction mixture will depend on the tissue samples. In retinal tissues with thickness of 7 μm, the recommended concentration from Kit was very strong and we have found the best dilution rate of the TUNEL reaction mixture is 1 (Enzyme solution) to 10 (Label solution).
Two negative controls and a positive control should be included in each experimental set up according to In Situ Cell Death Detection Kit. For negative control, the sections are incubated with 50 μL of Label solution instead of TUNEL reaction mixture. For positive control, the section is incubated with micrococal nuclease or DNase I recombinant (3000 U/mL-3 U/mL in 50 mM Tris-HCl, pH 7.5, 1 mg/mL bovine serum albumin, Roche Applied Science). The samples should be put under aluminum foil and the room lights dimmed for subsequent steps.
References
- 1.Ju WK, Lindsey JD, Angert M, Patel A, Weinreb RN. Glutamate receptor activation triggers OPA1 release and induces apoptotic cell death in ischemic rat retina. Mol Vis. 2008;14:2629–2638. [PMC free article] [PubMed] [Google Scholar]
- 2.Buchi ER. Cell death in the rat retina after a pressure-induced ischaemia-reperfusion insult: an electron microscopic study. I. Ganglion cell layer and inner nuclear layer. Exp Eye Res. 1992;55:605–613. doi: 10.1016/s0014-4835(05)80173-3. [DOI] [PubMed] [Google Scholar]
- 3.Nickells RW. Retinal ganglion cell death in glaucoma: the how, the why, and the maybe. J Glaucoma. 1996;5:345–356. [PubMed] [Google Scholar]
- 4.Rosenbaum DM, Rosenbaum PS, Gupta H, Singh M, Aggarwal A, Hall DH, Roth S, Kessler JA. The role of the p53 protein in the selective vulnerability of the inner retina to transient ischemia. Invest Ophthalmol Vis Sci. 1998;39:2132–2139. [PubMed] [Google Scholar]
- 5.Joo CK, Choi JS, Ko HW, Park KY, Sohn S, Chun MH, Oh YJ, Gwag BJ. Necrosis and apoptosis after retinal ischemia: involvement of NMDA-mediated excitotoxicity and p53. Invest Ophthalmol Vis Sci. 1999;40:713–720. [PubMed] [Google Scholar]
- 6.Ju WK, Kim KY, Hofmann HD, Kim IB, Lee MY, Oh SJ, Chun MH. Selective neuronal survival and upregulation of PCNA in the rat inner retina following transient ischemia. J Neuropathol Exp Neurol. 2000;59:241–250. doi: 10.1093/jnen/59.3.241. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Cho CH, Atchaneeyasakul LO, McFarland T, Appukuttan B, Stout JT. Activation of the mitochondrial apoptotic pathway in a rat model of central retinal artery occlusion. Invest Ophthalmol Vis Sci. 2005;46:2133–2139. doi: 10.1167/iovs.04-1235. [DOI] [PubMed] [Google Scholar]
- 8.Russo R, Rotiroti D, Tassorelli C, Nucci C, Bagetta G, Bucci MG, Corasaniti MT, Morrone LA. Identification of novel pharmacological targets to minimize excitotoxic retinal damage. Int Rev Neurobiol. 2009;85:407–423. doi: 10.1016/S0074-7742(09)85028-9. [DOI] [PubMed] [Google Scholar]
- 9.Szabo ME, Droy-Lefaix MT, Doly M, Carre C, Braquet P. Ischemia and reperfusion-induced histologic changes in the rat retina. Demonstration of a free radical-mediated mechanism. Invest Ophthalmol Vis Sci. 1991;32:1471–1478. [PubMed] [Google Scholar]
- 10.Hayreh SS, Weingeist TA. Experimental occlusion of the central artery of the retina. IV: Retinal tolerance time to acute ischaemia. Br J Ophthalmol. 1980;64:818–825. doi: 10.1136/bjo.64.11.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osborne NN, Larsen A, Barnett NL. Influence of excitatory amino acids and ischemia on rat retinal choline acetyltransferase-containing cells. Invest Ophthalmol Vis Sci. 1995;36:1692–1700. [PubMed] [Google Scholar]
- 12.Lombardi G, Moroni F. Glutamate receptor antagonists protect against ischemia-induced retinal damage. Eur J Pharmacol. 1994;271:489–495. doi: 10.1016/0014-2999(94)90810-9. [DOI] [PubMed] [Google Scholar]
- 13.Choi DW. Ischemia-induced neuronal apoptosis. Curr Opin Neurobiol. 1996;6:667–672. doi: 10.1016/s0959-4388(96)80101-2. [DOI] [PubMed] [Google Scholar]
- 14.Brandstatter JH, Koulen P, Wassle H. Diversity of glutamate receptors in the mammalian retina. Vision Res. 1998;38:1385–1397. doi: 10.1016/s0042-6989(97)00176-4. [DOI] [PubMed] [Google Scholar]
- 15.Brandstatter JH, Hack I. Localization of glutamate receptors at a complex synapse. The mammalian photoreceptor synapse. Cell Tissue Res. 2001;303:1–14. doi: 10.1007/s004410000304. [DOI] [PubMed] [Google Scholar]
- 16.Melino G. The Sirens’ song. Nature. 2001;412:23. doi: 10.1038/35083653. [DOI] [PubMed] [Google Scholar]
- 17.Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 18.Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nunez G, Peter ME, Tschopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]


