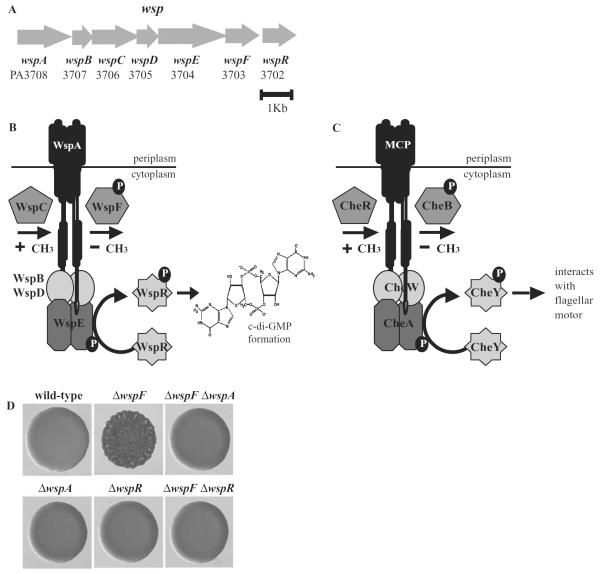
Fig. 1.
Characteristics of the Wsp signal transduction system.
A. Organization of the genes encoding the Wsp system.
B. The Wsp proteins are predicted to be a membrane-bound methyl-accepting chemotaxis protein (WspA), a CheR-like methyltransferase (WspC), a CheB-like methylesterase (WspF), two CheW homologues (WspB and WspD) that are predicted to serve as linkers between WspA and a hybrid histidine kinase response regulator (WspE). The response regulator protein, WspR, has a GGDEF domain and catalyses the synthesis of c-di-GMP when phosphorylated. As described in the text, a wspF mutation is predicted to lock the Wsp system into a configuration where WspR is constantly phosphorylated and thus constantly producing c-di-GMP.
C. The physical organization of homologous Che proteins (encoded by PA1456–1459, PA1464 and PA3349) and (D) colony morphologies of P. aeruginosa PAO1 wild-type and wsp deletion strains. wspF mutants have high levels of intracellular c-di-GMP relative to wild-type cells (Hickman et al., 2005).