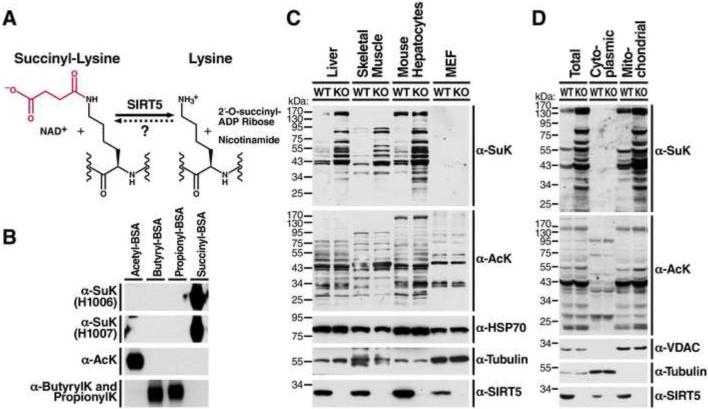
Figure 1. Generation of succinyl-lysine-specific antibodies and characterization of succinylation distribution in mouse tissues, cultured cells and subcellular compartments.
(A) The structure of lysine succinylation and the catalytic reaction of desuccinylation by SIRT5. The succinyl group on the lysine residue is indicated in red. (B) Succinyl-lysine antibodies specifically detected succinyl-BSA, but not acetyl-BSA or butyryl- or propionyl-BSA. (C) Western blot using succinyl-lysine-specific antibodies was performed to assess succinylation and acetylation levels in mouse liver, skeletal muscle, primary cultured mouse hepatocytes and MEFs derived from wild-type (WT) or Sirt5−/− mice (See also Figure S1A). Equal amount of proteins were loaded into each lane. Loading controls, α-tubulin and mitochondrial protein HSP70, were also examined. Western blot for SIRT5 confirmed the absence of SIRT5 protein in Sirt5−/− mouse tissues or cells. (D) Protein succinylation and acetylation in whole cell lysates, cytoplasmic or mitochondrial fractions derived from WT or Sirt5−/− mouse livers. Western blot for cytoplasmic protein α-tubulin and mitochondrial protein voltage dependent anion channel (VDAC) confirmed the purity of subcellular fractionation. Western blot for SIRT5 showed the presence of SIRT5 in both cytoplasm and mitochondria of WT livers, but not in Sirt5−/− livers.