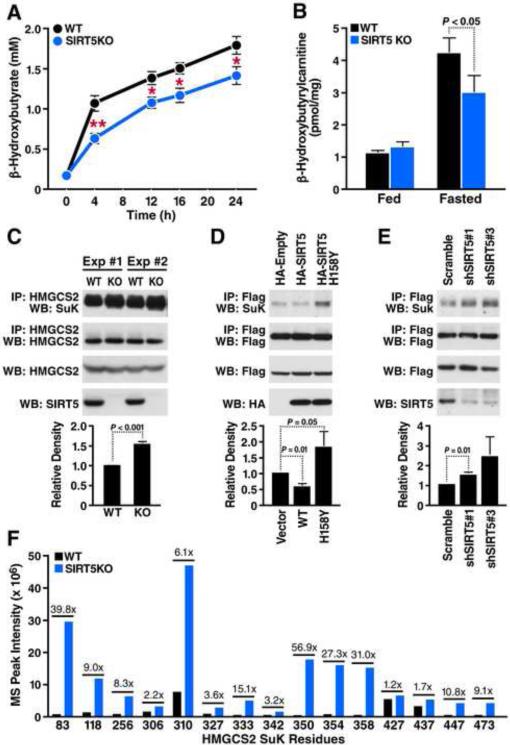
Figure 6. Lack of SIRT5 leads to decreased β-hydroxybutyrate production and hypersuccinylation of HMGCS2.
(A) Plasma β-hydroxybutyrate levels in WT and Sirt5−/− mice at 0, 4, 12, 16 and 24 hour following fasting (n=5, **p<0.001, *p<0.01). Results are shown as the mean ± standard error. (B) Liver β-hydroxybutyrylcarnitine levels in WT and Sirt5−/− mice under fed or 24 hour fasted condition (n=5). Results are shown as the mean ± standard error. (C) Western blot showing succinylation of endogenous HMGCS2 immunoprecipitated from WT or Sirt5−/− mouse liver (n=3). Integrated density values were calculated and are shown relative to WT mice. (D) Expression plasmids for WT HMGCS2 were transfected into HEK293 cells with an empty vector control, SIRT5 WT, or SIRT5 H158Y (catalytically inactive mutant). Immunoprecipitation and western blot were performed to examine succinylation levels of HMGCS2 (n=3). Integrated density values were calculated and are shown relative to empty vector control. (E) HEK293 cells over-expressing WT HMGCS2 were infected with lentivirus carrying scrambled shRNA or two different shRNAs for specifically knocking down SIRT5. Immunoprecipitation and western blot were performed to examine succinylation levels of HMGCS2 (n=5). Integrated density values were calculated and are shown relative to scrambled shRNA treatment. (F) Mass spectrum peak intensity of all identified succinyllysine-containing peptides measured in WT (black bars) and Sirt5−/− mice (blue bars). Fold change of each site (KO:WT) is indicated above the bars.