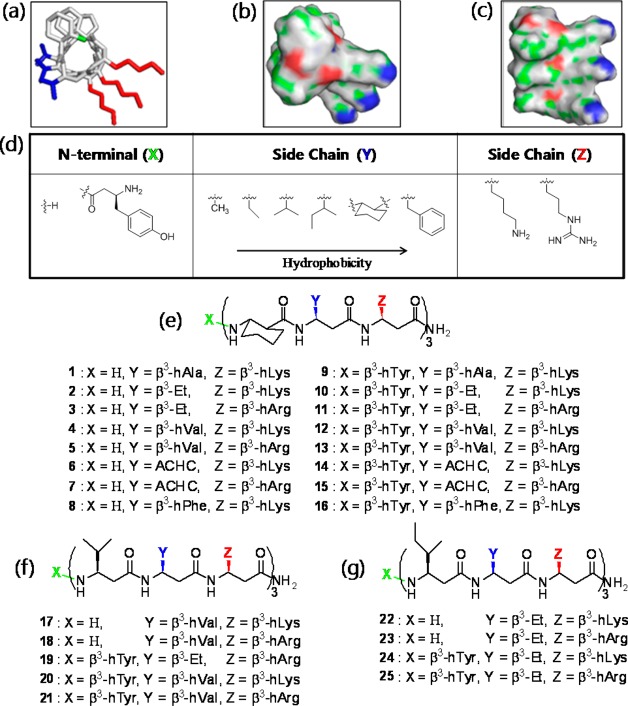
Figure 1.
14-Helical β-peptide design and chemical structures. 3D structures (a–c) were generated on the basis of available crystal structure data85 and then geometry was optimized using Gaussian 03 at the B3LYP/6-31G level. (a) Stick view of β-peptide 4. The N-terminus (green), hydrophobic side chains (blue), and cationic side chains (red) are indicated in color. (b and c) Surface views of β-peptide 4. Surface colors represent atom type H (gray), C (green), O (red), and N (blue). (d) N-Terminus (X) and side chains (Y and Z) were altered as indicated to vary peptide hydrophobicity. (e) Chemical structure of β-peptides containing a helix-stabilizing ACHC residue. (f and g) Chemical structures of β-peptides lacking an ACHC residue. β3-hVal (f) and β3-hIle (g) were incorporated in place of the ACHC residue.