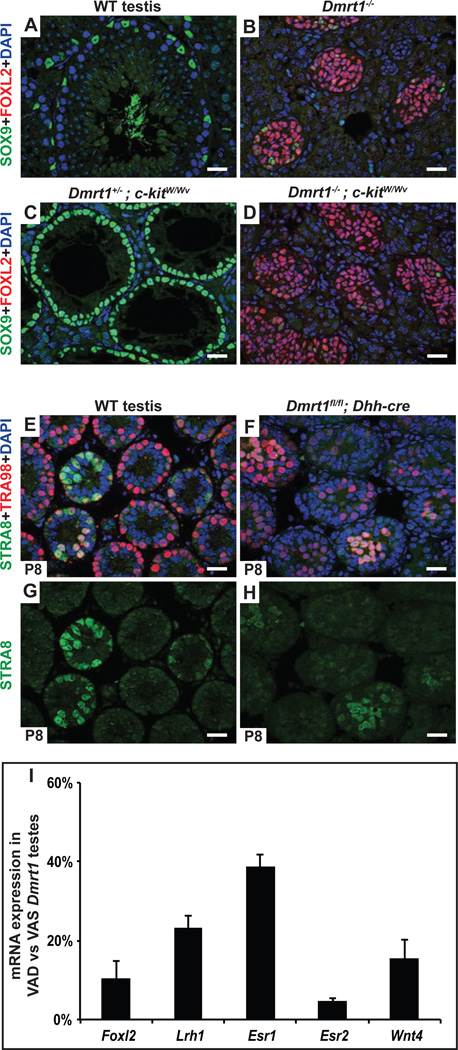
Figure 6. Feminizing RA is not produced by germ cells and appears to be at normal levels.
A-D, Germ cells are not required for transdifferentiation of Dmrt1 mutant Sertoli cells. Deletion of Dmrt1 causes most SOX9-positive Sertoli cells (A) to transdifferentiate into FOXL2 positive granulosa cells (B). Loss of c-kit function in eliminates virtually all germ cells during fetal development, leaving seminiferous tubules containing only differentiated SOX9-positive Sertoli cells in Dmrt1 heterozygous control testes (C). Testes of Dmrt1 homozygous kitW/Wv mutants were extensively feminized despite lacking germ cells (D). (Gonads in A-D were from adults). E-H, Loss of Dmrt1 in Sertoli cells does not induce STRA8 expression in spermatogonia. Expression of RA-inducible germ cell marker STRA8 and spermatogonial marker TRA98 in juvenile (P8) testes. In wild type testes (E,G) a subset of tubules contain germ cells with abundant STRA8 expression, reflecting the asynchronous initiation of spermatogenesis that is triggered by RA from Sertoli cells at this stage. When Dmrt1 is deleted in Sertoli cells (F,H) there is no obvious increase in the proportion of germ cells expressing STRA8 suggesting that intratubular RA levels are not elevated (RA is freely diffusible between cells). I, qRT-PCR showing reduced expression of feminizing gene mRNAs in VAD Dmrt1 mutants relative to VAS Dmrt1 mutants. Results are from two animals of each condition. (See also Fig. S3.)