Abstract
Purpose
The advantages of totally laparoscopic surgery in early gastric cancer (EGC) are unproven, and some concerns remain regarding the oncologic safety and technical difficulty. This study aimed to evaluate the technical feasibility and clinical benefits of totally laparoscopic distal gastrectomy (TLDG) for the treatment of gastric cancer compared with laparoscopy-assisted distal gastrectomy (LADG).
Materials and Methods
A retrospective review of 211 patients who underwent either TLDG (n=134; 63.5%) or LADG (n=77; 36.5%) for EGC between April 2005 and October 2013 was performed. Clinicopathologic features and surgical outcomes were analyzed and compared between the groups.
Results
The operative time in the TLDG group was significantly shorter than that in the LADG group (193 [range, 160~230] vs. 215 minutes [range, 170~255]) (P=0.021). The amount of blood loss during TLDG was estimated at 200 ml (range, 100~350 ml), which was significantly less than that during LADG, which was estimated at 400 ml (range, 400~700 ml) (P<0.001). The hospital stay in the TLDG group was shorter than that in the LADG group (7 vs. 8 days, P<0.001). One patient from each group underwent laparotomic conversion. Two patients in the TLDG group required reoperation: one for hemostasis after intraabdominal bleeding and 1 for repair of wound dehiscence at the umbilical port site.
Conclusions
TLDG for distal EGC is a technically feasible and safe procedure when performed by a surgeon with sufficient experience in laparoscopic gastrectomy and might provide the benefits of reduced operating time and intraoperative blood lossand shorter convalescence compared with LADG.
Keywords: Stomach neoplasms, Laparoscopy, Gastrectomy
Introduction
The incidence of gastric cancer in Korea was reported as 60.3 per 100,000 inhabitants in 2010, and the age-standardized incidence rate has been steady over the last decade. However, the 5-year relative survival rate has markedly improved from 42.8% to 67.0% primarily because of early detection via the national screening policy.1 As the incidence of early gastric cancer (EGC) is increasing, minimally invasive surgery using a laparoscopic approach has become a widely used procedure for the treatment of EGC in Korea. In addition, several centers are performing totally laparoscopic distal gastrectomy (TLDG) with intracorporeal reconstruction as the treatment of choice for distal EGC.
TLDG was first introduced in 1992 by Goh et al.2 demonstrating intracorporeal Billroth II anastomosis using laparoscopic linear staplers. Since then, the laparoscopic technique has steadily improved, and more effective staplers are being developed. However, to date, TLDG is not commonly performed because of the steep learning curve and concerns with the technical feasibility of the procedure. Here, we present our experiences and short-term surgical outcomes after TLDG with various types of intracorporeal anastomosis and compare TLDG with laparoscopy-assisted distal gastrectomy (LADG).
Materials and Methods
1. Patients
All consecutive patients who underwent laparoscopic gastrectomy (either LADG or TLDG) for gastric cancer at Soonchunhyang University Seoul Hospital between April 2005 and October 2013 were identified, and their medical records were retrospectively reviewed for the analysis.
All patients underwent preoperative assessment of disease extent using gastrofibroscopy and abdominal computed tomography. Laparoscopic gastrectomy was offered to patients who were preoperatively diagnosed with stage IA/IB gastric cancer according to the 7th edition American Joint Committee on Cancer staging system.3 Patients were classified into the following 2 groups: the TLDG group (patients who underwent laparoscopic distal gastrectomy with intracorporeal anastomosis) and the LADG group (patients who underwent laparoscopic distal gastrectomy with extracorporeal anastomosis). Demographics, clinicopathologic characteristics, and surgical outcomes were analyzed and compared between the groups. The surgical risk in patients was preoperatively assessed according to the American Society of Anesthesiologists (ASA) classification,4 as follows: I, healthy patient; II, mild systemic disease; III, severe systemic disease; IV, severe systemic disease that is a constant threat to life; and V, moribund patient unlikely to survive 24 hours with or without an operation. Operating time was defined as the time from skin incision to wound closure, and intraoperative blood loss was estimated from the amount of suctioned blood from the operative field that was described on the anesthetic chart. The severity of the postoperative complications was classified according to the Accordion Severity Grading System of Postoperative Complications.5
2. Surgical procedures
A single surgeon performed all surgeries. Five ports were used during the operative procedures and an additional trocar was occasionally placed in the subxiphoid area (5 mm, for the liver retractor) based on the anatomical differences in the patients' livers. The operative procedure was standardized with the performance of partial omentectomy and D1+ or greater lymphadenectomy based on the guidelines of the Japanese Research Society for Gastric Cancer.6,7 Before transecting the proximal margin of the stomach, we performed intraoperative gastrofibroscopy to determine the proximal resection margin (PRM) for all lesions except antral tumors. The proximal margin was marked with electrocautery on the stomach serosa under endoscopic monitoring.
3. Methods of intracorporeal anastomosis
1) Billroth II gastrojejunostomy with Braun anastomosis
A small incision was made on the posterior wall of the remnant stomach under the guidance of a bougie tube. Then, another small incision was made on the antimesenteric border of the proximal jejunum, 25~30 cm distal to the ligament of Treitz. Gastrojejunostomy was established using a linear stapler. The entry hole of the stapler was closed using an intracorporeal running suture technique. Finally, a side-to-side jejunojejunostomy was performed between the afferent and efferent loops using a linear stapler (Braun anastomosis).
2) Roux-en-Y gastrojejunostomy
After the gastrojejunal anastomosis was established as in the previously described Billroth II reconstruction, the proximal part of the jejunum was transected using the linear stapler. In case of an uncut Roux-en-Y reconstruction, a non-bladed stapler was used instead. Two small incisions were made on the antimesenteric border of the distal end of the biliopancreatic limb and on the jejunal Roux limb, 50 cm distal from the gastrojejunal anastomosis. Finally, a side-to-side jejunojejunostomy was constructed using a linear stapler in the same manner as the Braun anastomosis.
4. Methods of extracorporeal anastomosis
1) Billroth I gastroduodenostomy
In extracorporeal Billroth I anastomosis, either a side-to-end or end-to-end circular stapled gastroduodenostomy was performed as previously described in detail elsewhere.8 After lymph node dissection, an epigastric trocar incision was elongated for the extracorporeal procedure. The duodenum was transected after a purse-string clamp was applied and an anvil was inserted in the duodenal stump. The stomach was brought out of the abdominal cavity and transected at the PRM. The circular stapler was introduced into the remnant stomach and the posterior wall or greater curvature was pierced. The center rod of the stapler was then combined with the anvil head to accomplish gastroduodenostomy. The opening of the gastric wall was closed using a linear stapler.
2) Billroth II gastrojejunostomy with Braun anastomosis, Roux-en-Y gastrojejunostomy
The duodenum was transected intracorporeally. After lymph node dissection was completed, a 5-cm vertical midline incision was made by extending the subxiphoid port site. The fully mobilized stomach was delivered out of the abdominal cavity and anastomosis was performed extracorporeally. The basic procedures of extracorporeal anastomosis were the same as those of the previously described intracorporeal method. All anastomoses including gastrojejunostomy and jejunojejunostomy were performed using linear staplers, and the entry holes were hand-sewn closed.
5. Statistical analysis
Statistical analysis was performed using SPSS ver. 14.0 for Windows (SPSS Inc., Chicago, IL, USA). All values are expressed as mean±standard deviation except for the amount of blood loss, operative times, and days of hospital stay, which are expressed as median values and ranges. Patients' demographics and perioperative data were analyzed using the Pearson chi-square test, Fisher's exact test, or Student's t-test. Surgical outcomes were analyzed using the Wilcoxon rank sum test, Pearson chi-square test, or Fisher's exact test. A P-value of <0.05 was considered statistically significant.
Results
1. Demographics
Laparoscopic distal gastrectomy for distal gastric cancer was performed in 211 patients at our center during the study period. Seventy-seven (36.5%) patients were treated using the LADG approach and 134 (63.5%) patients were treated using the TLDG approach.
The clinical characteristics of the patients are shown in Table 1. There was no significant difference with regard to gender, age, body mass index, and previous intraabdominal operative history between the TLDG and LADG groups. The TLDG group had a significantly higher ASA classification score than the LADG group (P=0.010).
Table 1.
Patient demographics
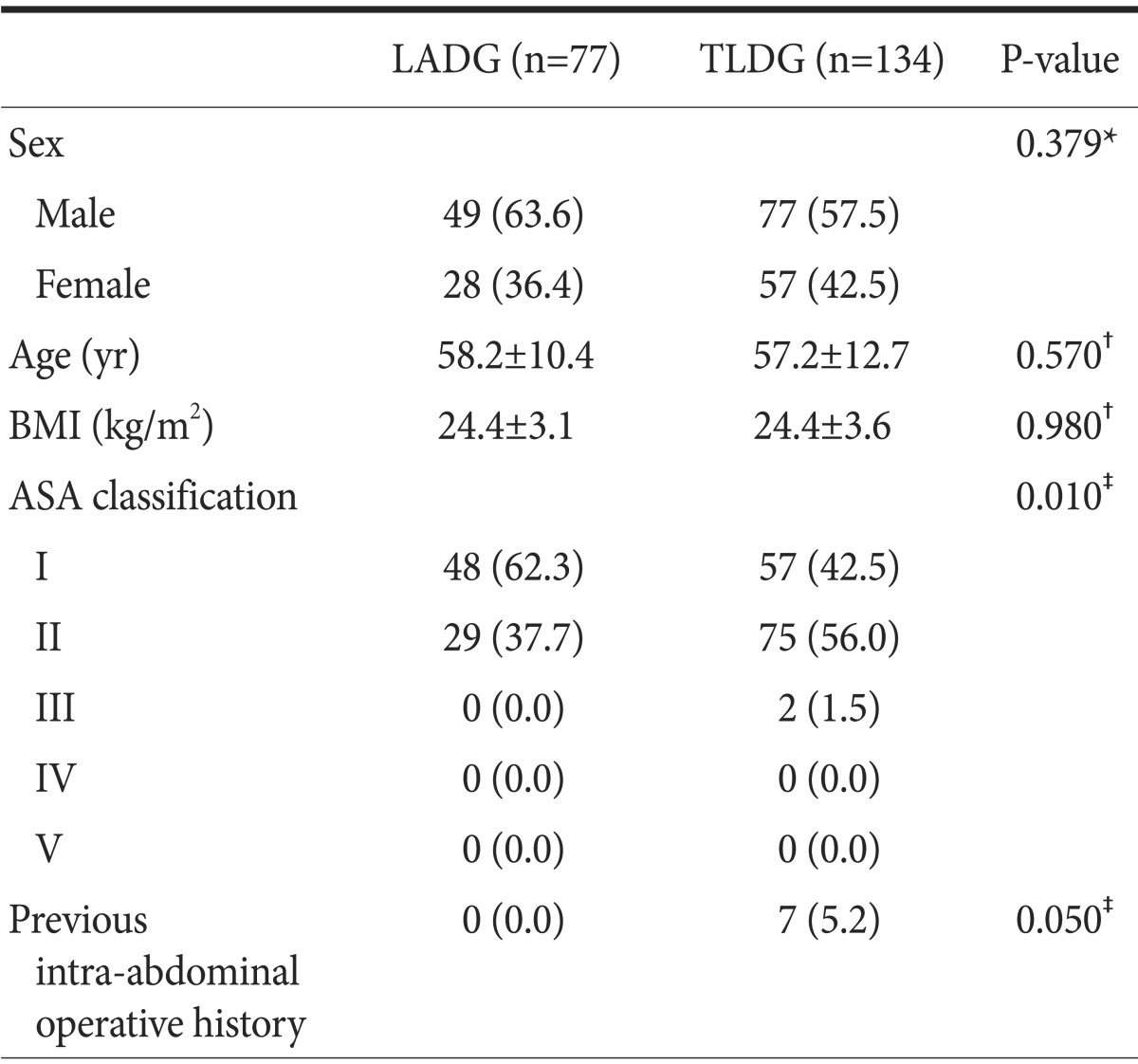
Values are presented as number (%) or mean±standard deviation. LADG = laparoscopy-assisted distal gastrectomy; TLDG = totally laparoscopic distal gastrectomy; BMI = body mass index; ASA = American Society of Anesthesiologists. *Pearson chi-square test.
†Student's t-test. ‡Fisher's exact test.
2. Surgical outcomes
The perioperative status of the patients is shown in Table 2. There was no significant difference between the TLDG and LADG groups with regard to combined operation and extent of lymph node dissection. However, the reconstructive procedures were significantly different between the 2 groups. In the LADG group, the Billroth I anastomosis was the most commonly used reconstructive method (39 of 77 patients, 50.6%), followed by the Billroth II with Braun anastomosis (29 of 77 patients, 37.7%), and finally the Roux-en-Y anastomosis (9 of 77 patients, 11.7%) for reconstruction after LADG. On the other hand, most patients in the TLDG group underwent either Billroth II with Braun anastomosis (47 of 134 patients, 35.1%) or Roux-en-Y anastomosis (87 of 134 patients, 64.9%).
Table 2.
Perioperative data of the patients
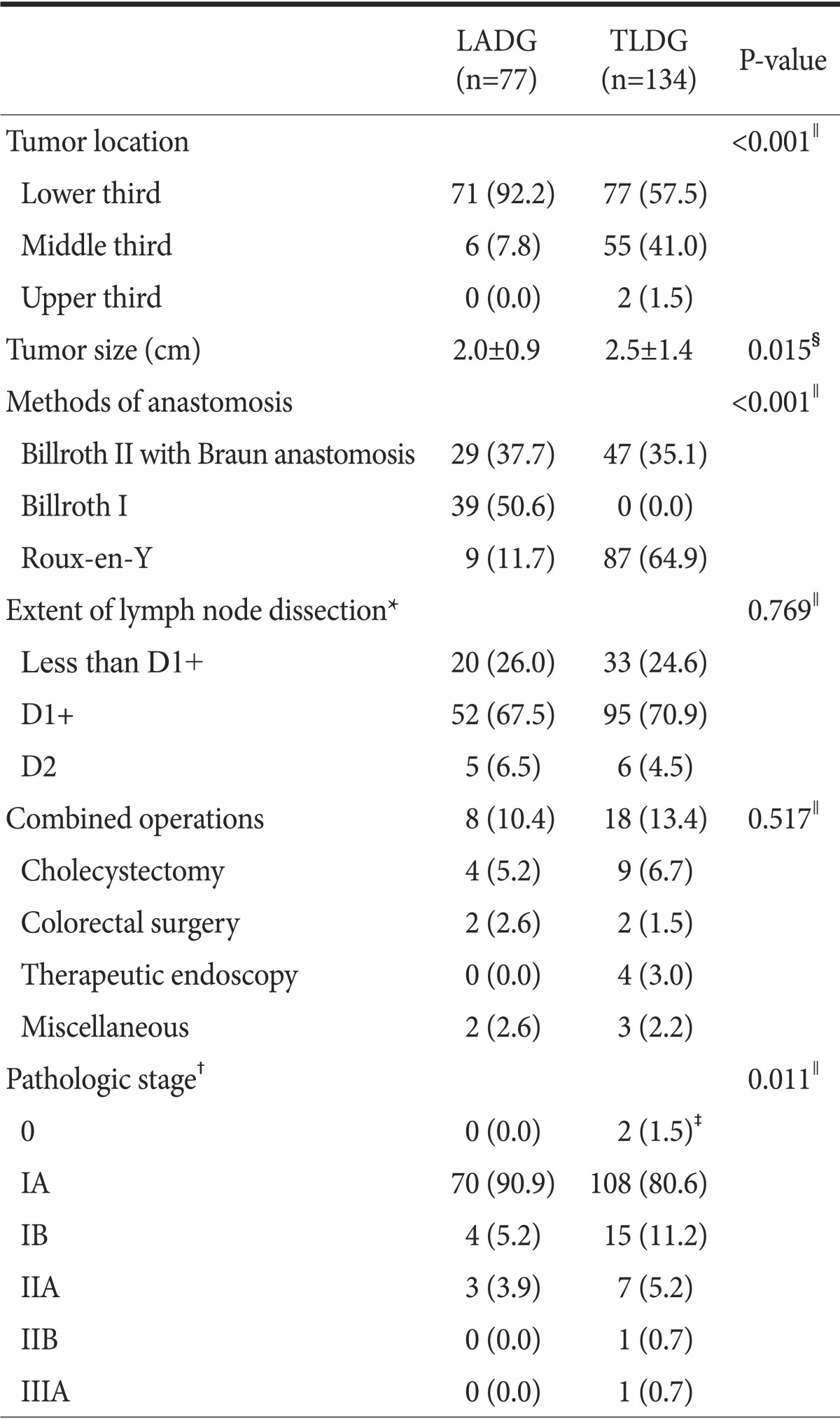
Values are presented as number (%) or mean¡¾standard deviation. LADG = laparoscopy-assisted distal gastrectomy; TLDG = totally laparoscopic distal gastrectomy. *Extent of lymph node dissection according to the Japanese gastric cancer treatment guidelines 2010 (ver. 3). †Pathological stage according to the 7th edition of the American Joint Committee on Cancer gastric cancer staging manual. ‡Two patients in the TLDG group who were preoperatively diagnosed with gastric cancer by endoscopic biopsy proved to have tubular adenoma with high-grade dysplasia after surgery. §Student's t-test. ∥Fisher's exact test.
The postoperative outcomes are shown in Table 3. The operative time in the TLDG group was significantly shorter than that in the LADG group (193 [range, 160~230] vs. 215 minutes [range, 170~255]) (P=0.021). The amount of blood loss during TLDG was estimated at 200 ml (range, 100~350 ml), which was significantly less than that during LADG, which was estimated at 400 ml (range, 400~700 ml) (P<0.001). The mean PRM in the TLDG group was significantly longer than that in the LADG group (5.7 vs. 4.0 cm, P=0.001). One patient in the LADG group underwent laparotomic conversion because of intraoperative bleeding from a ruptured right gastric artery. In the TLDG group, 1 patient underwent laparotomic conversion because of severe adhesion after iatrogenic perforation related to endoscopic submucosal dissection. Hospital stay in the TLDG group was shorter than that in the LADG group (7 vs. 8 days, P<0.001). After stratification of the overall postoperative complications based on their severity, the 2 groups were similar in the distribution of the severity of their postoperative complications (P=0.311). Severe complications developed in 3 patients in the TLDG group, with 2 of them requiring reoperation (1 for hemostasis related to intraabdominal bleeding and 1 for revision of wound dehiscence at the umbilical port under local anesthesia), and the other 1 requiring endoscopic intervention owing to luminal bleeding at the anastomosis site.
Table 3.
Postoperative outcomes of the patients
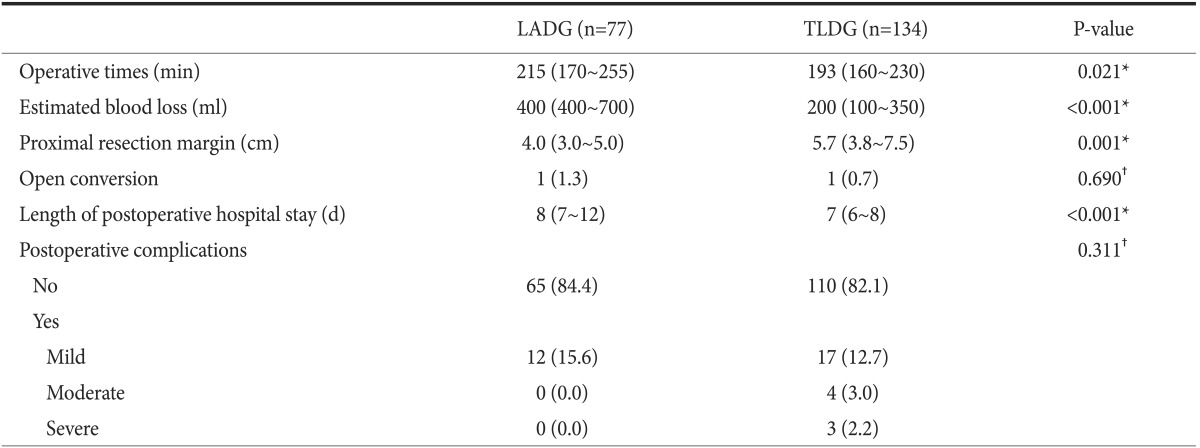
Values are presented as median (interquartile range) or number (%) unless otherwise indicated. LADG = laparoscopy-assisted distal gastrectomy; TLDG = totally laparoscopic distal gastrectomy. *Wilcoxon rank sum test. †Fisher's exact test.
Discussion
Since the introduction of LADG for EGC, many trials have evaluated the oncological feasibility and safety of LADG compared to those of conventional open distal gastrectomy (ODG). A prospective randomized multicenter study by Kim et al.9 suggested that LADG showed no significant difference in the morbidity and mortality compared with ODG. LADG showed similar 5-year disease-free survival and 5-year overall survival rates compared to those with ODG in the treatment of EGC. Additionally, LADG was associated with milder complications than ODG, based on an evaluation using the Accordion Severity Grading System of Postoperative Complications.10 Another randomized clinical trial revealed that LADG had contributed to improved quality of life in patients at the 3-month follow-up.11
Although TLDG was first introduced by Goh et al.2 in 1992, there is limited evidence regarding oncologic outcomes and its feasibility owing to the difficulty in skill acquisition and in experience. Previous studies have reported that at least 20~40 cases are needed to stabilize the surgical procedure for TLDG and to overcome the initial learning curve even for surgeons with sufficient experience in laparoscopic gastrectomy.12,13,14 Accordingly, even in Korea where the prevalence of EGC is high, only a few centers are performing TLDG for EGC. Only several studies have reported TLDG outcomes. Gao et al.15 published a meta-analysis showing that TLDG significantly reduced bleeding, time to first flatus, and postoperative hospital stay and can be considered a useful technique for patients with gastric cancer compared with LADG. Ikeda et al.16 suggested that TLDG had several advantages over LADG including smaller wounds, less invasiveness, and better feasibility of secure ablation. Song et al.17 reported that, compared with LADG, TLDG was more expensive but resulted in a shorter bowel recovery time, measured as the time to first flatus.
The present study showed results consistent with those of previous studies demonstrating that TLDG has advantages over LADG in terms of operative time, estimated blood loss, and postoperative hospital stay. The length of postoperative hospital stay in the TLDG group was shorter than that in the LADG group. This result suggests that the TLDG method may be less invasive, which is represented by smaller incisions and reduced manipulation. Furthermore, the shorter operative time and reduced estimated blood loss observed in the TLDG group support this postulation. The incidence of postoperative complications was similar between the groups, although the preoperative surgical risk, which is represented by the ASA classification, was higher in the TLDG group. These findings clearly suggest that TLDG has the benefit of earlier recovery over LADG, even in patients with a relatively high surgical risk.
In our study, the median PRM in TLDG was significantly longer than that in LADG despite the fact that the proportion of upper-middle lesions in the TLDG group was larger than that in the LADG group with statistical significance. This could have been influenced by the difference in reconstruction type and method between the groups. In LADG, the most frequently used reconstruction was Billroth I anastomosis (51.3%), followed by Billroth II with Braun anastomosis (36.8%). On the other hand, Billroth II with Braun anastomosis (35.1%) and Roux-en-Y anastomosis (64.9%) were used in most of the cases during TLDG because they were technically less demanding for the operating surgeon. In LADG cases, it is necessary to retain enough length of the remnant stomach to perform the extracorporeal anastomosis without difficulty. Furthermore, in LADG, the surgeon tended to secure a shorter proximal safe margin to reduce the tension in the anastomosis of the Billroth I reconstruction. Conversely, in TLDG, the surgeon tended to use a longer PRM because the EGC lesion could not be localized laparoscopically with palpation, resulting in the possibility of an unsatisfactory PRM even though simultaneous intraoperative endoscopy was used. Furthermore, the long remnant stomach in gastrojejunal anastomosis is not needed during intracorporeal anastomosis. As a result, TLDG could allow relatively sufficient and safe proximal margins, which could be easily applied for lesions located in the higher portion of the stomach, compared with LADG.
There are several limitations in the current study. First, surgeries were performed consecutively by a single surgeon, and TLDG was adopted relatively later than LADG. TLDG was first performed after gaining sufficient experience with 75 LADG cases, which may have resulted in a bias in the present study considering the learning curve associated with laparoscopic surgery. Second, different reconstruction types were used among the groups, which may have resulted in different surgical outcomes according to the reconstructive type. However, the number of enrolled patients in the present study was too small to conduct subgroup analyses based on the reconstruction types. Lastly, this study was retrospective in design, which may have introduced bias in the analyses. Detailed information about the surgical procedure and postoperative course, such as the time required for reconstruction, time to first flatus, and postoperative analgesic use, were also missing. A well-designed prospective study evaluating these parameters would be necessary to elucidate clearly the real benefits of intracorporeal anastomosis in laparoscopic distal gastrectomy.
In conclusion, TLDG is technically feasible and has several advantages over LADG, such as less intraoperative blood loss, shorter operative times and hospital stay, more sufficient PRM, and better cosmesis. TLDG could bea useful surgical technique for patients with EGC between the mid-upper body and the prepyloric area of the stomach when performed by a surgeon experienced in laparoscopic gastrectomy.
References
- 1.Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013;45:1–14. doi: 10.4143/crt.2013.45.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goh P, Tekant Y, Kum CK, Isaac J, Shang NS. Totally intra-abdominal laparoscopic Billroth II gastrectomy. Surg Endosc. 1992;6:160. doi: 10.1007/BF02309093. [DOI] [PubMed] [Google Scholar]
- 3.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 4.Wolters U, Wolf T, Stützer H, Schröder T. ASA classification and perioperative variables as predictors of postoperative outcome. Br J Anaesth. 1996;77:217–222. doi: 10.1093/bja/77.2.217. [DOI] [PubMed] [Google Scholar]
- 5.Strasberg SM, Linehan DC, Hawkins WG. The accordion severity grading system of surgical complications. Ann Surg. 2009;250:177–186. doi: 10.1097/SLA.0b013e3181afde41. [DOI] [PubMed] [Google Scholar]
- 6.Sano T, Aiko T. New Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised points. Gastric Cancer. 2011;14:97–100. doi: 10.1007/s10120-011-0040-6. [DOI] [PubMed] [Google Scholar]
- 7.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 8.Seo MW, Kim YJ, Song D, Kang GH, Cho GS, Lee MS, et al. Comparison of end-to-side and end-to-end anastomosis in circular stapled gastroduodenostomy. J Korean Gastric Cancer Assoc. 2009;9:57–62. [Google Scholar]
- 9.Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial) Ann Surg. 2010;251:417–420. doi: 10.1097/SLA.0b013e3181cc8f6b. [DOI] [PubMed] [Google Scholar]
- 10.Kim YW, Yoon HM, Yun YH, Nam BH, Eom BW, Baik YH, et al. Long-term outcomes of laparoscopy-assisted distal gastrectomy for early gastric cancer: result of a randomized controlled trial (COACT 0301) Surg Endosc. 2013;27:4267–4276. doi: 10.1007/s00464-013-3037-x. [DOI] [PubMed] [Google Scholar]
- 11.Kim YW, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008;248:721–727. doi: 10.1097/SLA.0b013e318185e62e. [DOI] [PubMed] [Google Scholar]
- 12.Kim HG, Park JH, Jeong SH, Lee YJ, Ha WS, Choi SK, et al. Totally laparoscopic distal gastrectomy after learning curve completion: comparison with laparoscopy-assisted distal gastrectomy. J Gastric Cancer. 2013;13:26–33. doi: 10.5230/jgc.2013.13.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahn CW, Hur H, Han SU, Cho YK. Comparison of intracorporeal reconstruction after laparoscopic distal gastrectomy with extracorporeal reconstruction in the view of learning curve. J Gastric Cancer. 2013;13:34–43. doi: 10.5230/jgc.2013.13.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim MC, Jung GJ, Kim HH. Learning curve of laparoscopy-assisted distal gastrectomy with systemic lymphadenectomy for early gastric cancer. World J Gastroenterol. 2005;11:7508–7511. doi: 10.3748/wjg.v11.i47.7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao J, Li P, Li QG, Chen J, Wang DR, Tang D. Comparison between totally laparoscopic and laparoscopically assisted distal gastrectomy for gastric cancer with a short follow-up: a meta-analysis. J Laparoendosc Adv Surg Tech A. 2013;23:693–697. doi: 10.1089/lap.2012.0580. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda O, Sakaguchi Y, Aoki Y, Harimoto N, Taomoto J, Masuda T, et al. Advantages of totally laparoscopic distal gastrectomy over laparoscopically assisted distal gastrectomy for gastric cancer. Surg Endosc. 2009;23:2374–2379. doi: 10.1007/s00464-009-0360-3. [DOI] [PubMed] [Google Scholar]
- 17.Song KY, Park CH, Kang HC, Kim JJ, Park SM, Jun KH, et al. Is totally laparoscopic gastrectomy less invasive than laparoscopy-assisted gastrectomy?: prospective, multicenter study. J Gastrointest Surg. 2008;12:1015–1021. doi: 10.1007/s11605-008-0484-0. [DOI] [PubMed] [Google Scholar]


