Abstract
Background
Considerable debate exists as to appropriate perioperative fluid management. Data from several studies suggest that the amount of fluid administered perioperatively influences surgical outcome. Pancreatic resection is a major procedure in which complications are common. We examined 1,030 sequential patients who had undergone pancreatic resection at Memorial Sloan-Kettering Cancer Center. We documented the prevalence and nature of their complications, and then correlated complications to intraoperative fluid administration.
Methods
We retrospectively examined 1,030 pancreatic resections performed at Memorial Sloan-Kettering Cancer Center between May 2004 and December 2009 from our pancreatic database. Intraoperative administration of colloid and crystalloid was obtained from anesthesia records, and complication data from our institutional database.
Results
The overall in-hospital mortality was 1.7%. Operative mortality was due predominantly to intraabdominal infection. Sixty percent of the mortality resulted from intraabdominal complications related to the procedure. We did not demonstrate a clinically significant relationship between intraoperative fluid administration and complications, although minor statistical significance was suggested.
Conclusions
In this retrospective review of intraoperative fluid administration we were not able to demonstrate a clinically significant association between postoperative complications and intraoperative crystalloid and colloid fluid administration. A randomized controlled trial has been initiated to address this question.
Keywords: pancreatic resection, intraoperative fluid, complications
INTRODUCTION
Considerable debate exists as to the appropriate perioperative fluid management for patients undergoing major intraabdominal surgery, which elicits a pronounced stress response. There remain proponents for the liberal use of fluid, and those who would advocate a more restrictive approach [1, 2].
Data from several studies suggest that the amount of fluid administered perioperatively influences surgical outcome and may be procedure dependent [3, 4]. Restricting fluids has been shown to reduce cardiac and pulmonary complications, anastomotic leaks [5, 6], promote early extubation [7] and decrease time to return of gastrointestinal (GI) function and length of hospital stay [1, 8]. Data from postoperative studies also suggest that restricting fluid after operation improves gastric emptying, decreases hospital stay [9], and decreases other complications [10].
Conversely, a randomized trial of fluid management after colorectal surgery suggested that less than 2 L, versus the standard greater than 3 L in 24 hr, did not affect time to flatus, return of bowel function or length of hospital stay, a somewhat prolonged 7.2 days [11].
The few studies looking at fluid management in pancreatic resection are divided between those supporting the restricted approach—fewer complications, quicker return of GI function and shorter hospital stay (n = 29) [1], and those finding no difference in postoperative bleeding, wound infection, pancreatic fistula, and mortality with larger amounts of fluids (n = 98) [2].
Pancreatic resection is a major procedure in which complications are common. Overall complication rates vary between 38% and 58%, most commonly pancreatic anastomotic leak, fistula, wound infection, and delayed gastric emptying. All of these morbidities are associated with increased length of stay [12, 13]. While mortality has markedly decreased, the nature and prevalence of such complications has changed little in 60 years [14, 15], although recognition and treatment have improved.
We initiated the present study in light of three observations.
The known controversy outlined above.
Our recent experience at Memorial Sloan-Kettering Cancer Center with hemodilution during major pancreatic resection in 130 patients suggesting that in those patients who underwent hemodilution there was a trend towards increased complications postoperatively [16]. The increase in complications was associated with the increased crystalloid load given to ensure the safety of hemodilution.
Our need for information to decide if a randomized controlled trial was justified.
In an effort to dissect this question, we examined a large series of sequential patients who had undergone pancreatic resection. We comprehensively documented the prevalence and nature of their complications, and then correlated complications to intraoperative fluid intake.
MATERIALS AND METHODS
The study was approved by the Institutional Review Board.
We retrospectively examined 1,030 pancreatic resections performed at Memorial Sloan-Kettering Cancer Center between May 2004 and December 2009 from a prospective database and analyzed a subgroup of 577 patients who underwent resection for pancreatic adenocarcinoma. Intraoperative administration of colloid and crystalloid was obtained from anesthesia records. In our institution, anesthesia is provided intraoperatively by a nurse anesthetist or anesthesia resident, supervised by Board Certified Anesthesiologists. Standard management approaches to fluid replacement and resuscitation are guided by the magnitude and duration of the procedure, patient comorbidities and the extent of fluid and blood loss. Preoperative mechanical bowel preparation is not used [17]. Initial blood loss in stable patients with adequate hemoglobin at the commencement of the procedure is replaced with either crystalloid 3:1 or colloid (5% albumin). Maintenance crystalloid is provided at approximately 750–1,000 ml/hr with adjustments made for body weight/BMI and patient comorbidities by the supervising anesthesiologist. A specific fluid regimen was not employed. Patients with advanced age, lower body weights, and reduced cardiac function were more likely to receive fluids on the lower range of the scale, whereas patients with higher starting bilirubin levels were given more fluid. Transfusion threshold at our institution is 8 g/dl in the absence of hemodynamic instability. The vast majority of these cases were supervised by two anesthesiologists assigned to the Hepatopancreaticobiliary Service. We gathered complication prevalence from our institutional database. Complications were graded one through five and complications within 90 days of resection were recorded [13]. In-hospital mortality was documented. We examined all of the complications and those that were grade three or greater, that is, complications that required a significant major intervention, prolonged morbidity, or resulted in death [13].
Statistical Analysis
We used Spearman’s rank correlation to analyze the correlations between fluid use, blood loss, and length of stay. Associations between complications were reported using summary statistics of these variables for patients who did and did not have major (grade 3 or greater) complications, separately. These values were compared using a Wilcoxon rank-sum test. The analysis of the subset of patients with adenocarcinomas followed the same plan. The confidence intervals for the difference in medians between groups were estimated using the accelerated and bias corrected bootstrap method.
RESULTS
We examined 1,030 patients who underwent 679 pancreaticoduodenectomies, 316 distal pancreatectomies, 13 total pancreatectomies, 12 central pancreatectomies, and 10 other procedures. Complications that occurred in greater than 1% of patients are listed in Table I. Complications listed under “other” are included as Appendix A. Mortality is also included in Table I. Complications are listed as occurrences and most patients who had a complication had more than one. Generic complications are summarized in Appendix B with the details included in Appendix C. The overall in-hospital mortality was 1.7%; operative mortality was due predominantly to intraabdominal infection or co-morbidity. Sixty percent of the mortality resulted from intraabdominal complications related to the procedure. The most common complications were wound infection (9%) and an anastomotic leak with or without subsequent intraabdominal infection or abscess (15%). Delayed gastric emptying occurred in 3% of patients. Not surprising, complications were interrelated.
TABLE I.
Complications (n = 1,030 Patients)
| # of occurrencesa | %b | |
|---|---|---|
| Complication name | ||
| Wound infection | 96 | 9.3 |
| Anastomotic leak, pancreatic | 83 | 8.1 |
| Intra-abdominal infection or abscess | 67 | 6.5 |
| Delayed gastric emptying (DGE) | 29 | 2.8 |
| Supraventricular arrhythmia | 28 | 2.7 |
| Pulmonary embolus | 20 | 1.9 |
| Hemorrhage | 19 | 1.8 |
| Urinary tract infection | 19 | 1.8 |
| Death | 18 | 1.7 |
| Non infected intra-abdominal/intra-thoracic fluid collection | 16 | 1.6 |
| Fistula, pancreatic | 14 | 1.4 |
| Other | 156 | 15.4 |
| Mortality (in-hospital) | 18 | 1.7% |
| Primary cause: | ||
| Leak/abscess/sepsis | 7 | 0.68 |
| Pulmonary embolus | 3 | 0.19 |
| Myocardial infarction | 2 | 0.19 |
| Ventricular arrhythmia | 2 | 0.19 |
| Visceral artery thrombosis | 2 | 0.19 |
| Gastrointestinal (GI) bleed | 1 | 0.1 |
| Aspiration | 1 | 0.1 |
Many patients had >1 complication.
% patients with that complication.
The measured perioperative variables (mean and median) are indicated for the entire cohort in Table II. Median blood loss was 500 ml, median duration of operation was 236 min and total fluid administered was four liters, the majority of which was crystalloid. When we examined only those patients who underwent resection for pancreatic adenocarcinoma (Table II), the measured variables were similar, that is, significant differences were not encountered based on histopathological diagnosis. Operations required similar periods of time whether for adenocarcinoma, other malignancies, or benign disease.
TABLE II.
Measured Variables for all Resections (n = 1,030) and for Resection for Adenocarcinoma (n = 577)
| Variable | Mean ± SD (All) | Median (range) (All) | Mean ± SD (Adeno) | Median (range) (Adeno) |
|---|---|---|---|---|
| Estimated blood loss (ml) | 676 ± 760 | 500 (0–8,500) | 730 ± 707 | 600 (0–8,500) |
| Length of stay (days) | 10.5 ± 12.4 | 8 (1–252) | 11.5 ± 14.0 | 9 (1–252) |
| Total fluids (ml) | 4,246 ± 1,839 | 4,000 (700–13,000) | 4,508 ± 1,832 | 4,300 (1,100–11,700) |
| Colloid (ml) | 307 ± 450 | 0 (0–3,000) | 359 ± 475 | 0 (0–3,000) |
| Crystalloid (ml) | 3,939 ± 1,607 | 3,700 (700–12,000) | 4,149 ± 1,593 | 4,000 (1,100–10,000) |
| Surgical time (min) | 244 ± 84 | 236 (49–558) | 264 ± 79 | 261 (70–558) |
Table III examines the correlations among these measured parameters. Estimated blood loss correlated significantly with the total fluids provided both for colloid and crystalloid. The surgical time and the length of hospital stay for all patients were inter-related with all five factors. Increased operative time resulted in increased crystalloid administration. Increased colloid is associated with increased blood loss as it is our practice to replace some of the initial blood loss with colloid in stable patients with adequate preoperative hemoglobin.
TABLE III.
Correlation Coefficients for all Resections (n = 1,030)
| Estimated blood loss | Length of stay | Total fluids | Colloid | Crystalloid | Surgical time | |
|---|---|---|---|---|---|---|
| Estimated blood loss (ml) | 1.0 | 0.15 <0.0001 | 0.65 <0.0001 | 0.47 <0.0001 | 0.62 <0.0001 | 0.32 <0.0001 |
| Length of stay (days) | 0.15 <0.0001 | 1.0 | 0.16 <0.0001 | 0.18 <0.0001 | 0.13 <0.0001 | 0.15 <0.0001 |
| Total fluids (ml) | 0.65 <0.0001 | 0.16 <0.0001 | 1.0 | 0.60 <0.0001 | 0.97 <0.0001 | 0.61 <0.0001 |
| Colloid (ml) | 0.47 <0.0001 | 0.18 <0.0001 | 0.60 <0.0001 | 1.0 | 0.41 <0.0001 | 0.33 <0.0001 |
| Crystalloid (ml) | 0.62 <0.0001 | 0.13 <0.0001 | 0.97 <0.0001 | 0.41 <0.0001 | 1.0 | 0.60 <0.0001 |
| Surgical time (min) | 0.32 <0.0001 | 0.15 <0.0001 | 0.61 <0.0001 | 0.33 <0.0001 | 0.60 <0.0001 | 1.0 |
In the patients undergoing resection for adenocarcinoma, there were clear correlations between the extent of blood loss, operative time, colloid fluid replacement, and length of stay for this group (details in Appendix D). While length of stay was significantly prolonged by the presence of a complication, the statistically significant (P < 0.001) increase in total fluids and colloid replacement between those with and without a complication was clinically of minimal relevance with median differences in total fluids of 300 ml (7.5%) and no change in median colloid administration between those with and without a complication. It is impossible to dissect such small differences in mean and median in such a large sample.
In order to examine whether or not fluid volume administration was related to complications, we compared fluid administration in patients with grade 3 or greater complications to administration in those without complications or with grades 1 and 2 complications (Table IV). When the entire cohort was examined, no difference in intraoperative crystalloid administration could be identified between those with and without complications. Patients who had complications (Table IV) had prolonged length of stay, increased colloid administration and increased total fluid administered.
TABLE IV.
Measured Variables for all Patients (A) and for Patients With ≥Grade 3 Complications (B) With and Without Complications
| Mean ± SD | Median (range) | Mean ± SD | Median (range) | ||
|---|---|---|---|---|---|
| (A) ALL | No (n = 821) | No (n = 821) | Yes (n = 209) | Yes (n = 209) | P |
| Length of stay (days) | 8.4 ± 4.7 | 8 (1–68) | 19.1 ± 24.2 | 12 (1–252) | <0.001 |
| Total fluids (ml) | 4,173 ± 1,769 | 3,900 (700–13,000) | 4,533 ± 2,069 | 4,200 (1,000–11,350) | 0.038 |
| Colloid (ml) | 281 ± 425 | 0 (0–3,000) | 407 ± 525 | 0 (0–2,500) | 0.002 |
| Crystalloid (ml) | 3,891 ± 1,554 | 3,700 (700–12,000) | 4,126 ± 1,793 | 3,800 (1,000–10,000) | 0.142 |
| Mean ± SD | Median (range) | Mean ± SD | Median (range) | ||
| (B) >Grade 3 | No (n = 458) | No (n = 458) | Yes (n = 119) | Yes (n = 119) | P |
| Length of stay (days) | 9.0 ± 4.8 | 8 (3–63) | 21.1 ± 27.5 | 14 (1–252) | <0.001 |
| Total fluids (ml) | 4,467 ± 1,765 | 4,200 (1,100–11,700) | 4,667 ± 2,070 | 4,400 (1,200–11,350) | 0.425 |
| Colloid (ml) | 344 ± 463 | 0 (0–3,000) | 416 ± 518 | 250 (0–2,500) | 0.232 |
| Crystalloid (ml) | 4,123 ± 1,527 | 4,000 (1,100–10,000) | 4,250 ± 1,828 | 4,000 (1,200–10,000) | 0.722 |
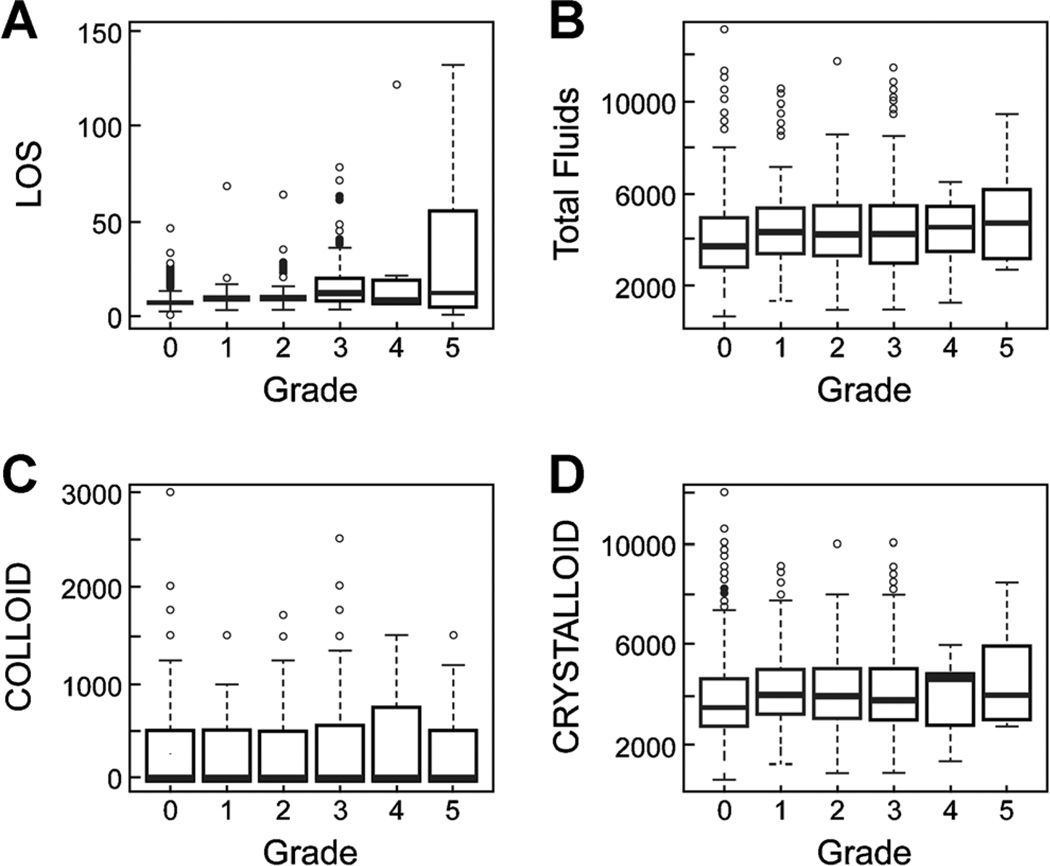
As fluid administration may have influenced grades 1 and 2 complications, in Figure 1, we show complications for all grades in terms of length of stay, total fluids, and colloid and crystalloid administration. There was wide variation but no clear clinically significant associations.
Fig. 1.
Measured variables by grade of complication. A: Length of stay (LOS), B: Total fluids, C: Colloid, D: Crystalloid.
When we examined complications by grade versus length of stay, an increase in grade of complication did increase length of stay, but there were insufficient grades 4 and 5 complications to be meaningful. Death prior to discharge, if anything, may abbreviate hospital stay. The trend, however, is statistically significant (Appendix E).
DISCUSSION
The natural response to injury or operation is fluid and electrolyte retention. Fluid retention persists for a variable time until mobilization and diuresis occur. Many principles of perioperative fluid management stem from work done in the 1950s. Francis Moore argued for perioperative fluid restriction in 1959 citing the diminished ability to excrete excess salt and water due to the metabolic and endocrine response to tissue trauma [18].
Shires, in an editorial written with Moore in 1967 examining his own recommendations to use salt solutions to fill and maintain vascular volume by flooding the interstitial fluid volume, stated that the approach does not apply to simple blood loss. They recommended that fluid replacement during operation should be carefully estimated and limited unless there is additional excessive measurable blood loss, using a balanced salt solution as a “physiological adjunct” to surgical trauma, not a substitute for blood [19].
In the following decades, large amounts of fluids continued to be recommended to correct perioperative deficits, support the circulation and preserve urine output in surgery, trauma, and the critically ill [20–28]. These earlier studies placed emphasis on intraoperative fluid shifts resulting in hidden fluid loss and intravascular hypovolemia. The decrease in venous return, systemic vascular resistance and myocardial contractility during induction of anesthesia, depression of the endogenous sympathoadrenal mechanism, reduction of preload during positive pressure ventilation, and sympathetic nervous blockade from regional anesthesia causing vasodilation were all cited as causes for need for increased intraoperative fluid [22].
Previous studies have suggested that the liberal use of fluid can increase perioperative complications. Hypervolemia can lead to decreased pulmonary function [29, 30], inhibited gut motility [1, 9, 31], increased postoperative ileus [9], decreased subcutaneous oxygen tension [32], pulmonary edema [5, 33], potential impairment of left ventricular stroke volume [31], myocardial ischemia [31], atelectasis, pneumonia, and respiratory failure [31, 33], increased excretory work of the kidneys [31, 34], coagulation abnormalities [31], delayed wound healing [31], decreased tissue oxygen tension [31, 32], postoperative edema and weight gain [35], anastomotic leaks [27], and wound dehiscence [26]. Conversely, hypovolemia is associated with a decrease in perfusion and an increase in complications, although numbers are small (n = 70), and the patients with pancreatectomy are few (n = 14) [36].
A positive association between liberal fluid management and postoperative complications has been demonstrated in prospective randomized trials in colorectal cancer and in other intraabdominal operations [1, 37, 38]. We hypothesized that as complications are commonly seen in pancreatic resection, this was worthy of study. Serious complications are as high as 40%, as we have previously reported [13].
The complications we experienced were similar to those reported from our own institution in 2003 where 204 patients were analyzed [13]. The anastomotic leak rate from the pancreas appeared to decrease but overall, leak, and intraabdominal abscess continue to occur in approximately 17% of patients. The ability to determine the true source of an intraabdominal abscess with pancreatic or biliary leak remains difficult. Delayed gastric emptying, which was reported in 7% of our previous cases is reported on this occasion in 3%. Definitions of delayed gastric emptying remain variable, and with decreased use of nasogastric suction, may result in under appreciation of its prevalence.
When we looked at grouping of complications both one through five and >3 and related those to operative fluid administration along with documentation of estimated blood loss, surgical time, and length of stay, complications were strongly associated with increased length of stay [1, 9, 39].
The difference for total fluids between those who had any complication and those who did not was statistically significant (P = 0.038, Table IV). Despite this significance, the median difference in total fluids between the two groups is 300 ml and the 95% confidence interval for this difference is 50–700 ml. Hence the largest median difference between these two groups supported by our data is 700 ml. We do not consider this difference clinically meaningful and attribute the statistical significance to our large sample size. Additional support for this conclusion can be found in the analysis of the subset of patients who had adenocarcinomas. The statistical significance in this smaller subset disappears, although the median difference is comparable at 200 ml.
The hypothesis that total fluid given intraoperatively can predict or be causatively associated with complications cannot be directly answered by this study as the total fluids administered are predicated on the variables that are created by length of operation and blood loss, that is, increasing colloid use occurs when there is increased blood loss as initial blood loss is replaced by crystalloid, and then by colloid, prior to blood administration. Total fluid administration is dependent on the extent of blood loss and surgical time. Factors that correlate with length of stay, which in itself is highly dependent on the presence or absence of a complication, are inherently encompassed by intraoperative blood loss and surgical time, both of which by standard approaches to intraoperative fluid administration result in increased intraoperative fluid delivery.
The limitations of the present study primarily devolve around the examination of intraoperative fluid only. Prior studies have suggested that it is total perioperative fluid rather than intraoperative fluid which affects surgical outcome [1, 9, 37–40]. Despite the large sample size derived from a prospective data base, complications are derived from a second administrative data base of all postoperative complications, and are not specific to pancreatic surgery.
CONCLUSIONS
We confirm and extensively document the significant complications seen following pancreaticoduodenectomy. In situations where intraoperative and perioperative fluid replacement is based on algorithms dependent mainly on time and fluid loss, it is difficult to examine the influence of fluid administration on subsequent complications. A prospective randomized trial is underway in patients undergoing pancreatectomy, where fluid administration is randomized to one of two specific regimens, restrictive or liberal, based on body weight and BMI, and not dependent solely on duration of procedure. The regimens are continued through the PACU stay and onto the floor until the patients meet one of several milestones. Complication data will continue to be collected prospectively and from our institutional database.
APPENDIX A. Complications, Other (<1%)
| Anastomotic leak, biliary | 10 | 0.97 |
| Clostridium difficile colitis | 10 | 0.97 |
| Gastrointestinal bleed | 10 | 0.97 |
| Deep venous thrombosis | 9 | 0.87 |
| Ileus, paralytic | 9 | 0.87 |
| Cerebrovascular accident | 8 | 0.78 |
| Pneumonitis | 8 | 0.78 |
| Ventricular arrhythmia | 7 | 0.68 |
| Ascites | 6 | 0.58 |
| Hematoma | 6 | 0.58 |
| Sepsis | 6 | 0.58 |
| Fascial dehiscence or evisceration | 5 | 0.49 |
| Vascular thrombosis | 5 | 0.49 |
| Wound breakdown | 5 | 0.49 |
| Pleural effusion | 4 | 0.39 |
| Renal failure | 4 | 0.39 |
| Respiratory failure | 4 | 0.39 |
| Small bowel obstruction | 4 | 0.39 |
| Urinary retention | 4 | 0.39 |
| Aspiration | 3 | 0.29 |
| Congestive heart failure, left ventricular dysfunction | 3 | 0.29 |
| Fistula, intestinal | 3 | 0.29 |
| Hypotension, shock | 3 | 0.29 |
| Myocardial infarction | 3 | 0.29 |
| Seroma | 3 | 0.29 |
| Anastomotic leak, intestinal | 2 | 0.19 |
| Cholangitis | 2 | 0.19 |
| Multi organ system failure | 2 | 0.19 |
| Pancreatitis | 2 | 0.19 |
| Acute respiratory distress syndrome | 1 | 0.1 |
| Catheter related infection | 1 | 0.1 |
| Fistula, biliary | 1 | 0.1 |
| Large bowel obstruction | 1 | 0.1 |
| Liver failure | 1 | 0.1 |
| Visceral artery ischemia | 1 | 0.1 |
APPENDIX B. Complications by Group (n = 1,030 Patients)
| Complication name | # of occurrences | %a |
|---|---|---|
| Wound | 106 | 10.3 |
| Leak | 180 | 17.6 |
| Delayed gastric emptying | 29 | 2.8 |
| Other GI tract | 34 | 3.3 |
| Cardiac | 41 | 4 |
| Pulmonary embolus | 20 | 1.9 |
| Hemorrhage | 19 | 1.8 |
| Urinary tract | 23 | 2 |
| Intraabdominal/intrathoracic fluid collection | 36 | 2.6 |
| Deep vein thrombosis (DVT) | 9 | 0.9 |
| Cerebral vascular accident (CVA) | 8 | 0.8 |
| Pulmonary/respiratory | 16 | 1.6 |
| Organ failure/sepsis | 16 | 1.6 |
| Other | 20 | 1.9 |
| Death | 18 | 1.7 |
% of patients with that complication.
APPENDIX C. Complications by Group (n = 1,030 Patients)
| # of occurrences | %a | |
|---|---|---|
| Wound | 106 | 10.3 |
| Infection | 96 | 9.3 |
| Fascial dehiscence or evisceration | 5 | 0.5 |
| Wound breakdown | 5 | 0.5 |
| Leak | 180 | 17.6 |
| Anastomotic leak, pancreatic | 83 | 8.1 |
| Intraabdominal infection or abscess | 67 | 6.5 |
| Fistula, pancreatic | 14 | 1.4 |
| Anastomotic leak, biliary | 10 | 0.97 |
| Fistula, intestinal | 3 | 0.29 |
| Anastomotic leak, intestinal | 2 | 0.19 |
| Fistula, biliary | 1 | 0.1 |
| Delayed gastric emptying | 29 | 2.8 |
| Other gastrointestinal tract | 34 | 3.3 |
| Clostridium difficile colitis | 10 | 0.97 |
| Gastrointestinal bleed | 10 | 0.97 |
| Ileus, paralytic | 9 | 0.87 |
| Small bowel obstruction | 4 | 0.39 |
| Large bowel obstruction | 1 | 0.1 |
| Cardiac | 41 | 4 |
| Supraventricular arrhythmia | 28 | 2.7 |
| Ventricular arrhythmia | 7 | 0.68 |
| Congestive heart failure, LV (left ventricular) dysfunction | 3 | 0.29 |
| Myocardial infarction | 3 | 0.29 |
| Pulmonary embolus | 20 | 1.9 |
| Hemorrhage | 19 | 1.8 |
| Urinary tract | 23 | 2 |
| Infection | 19 | 1.8 |
| Urinary retention | 4 | 0.39 |
| Intraabdominal/intrathoracic fluid collection | 36 | 2.6 |
| Non infected | 16 | 1.6 |
| Ascites | 6 | 0.58 |
| Pleural effusion | 4 | 0.39 |
| Deep vein thrombosis | 9 | 0.9 |
| Cerebral vascular accident | 8 | 0.8 |
| Pulmonary/Respiratory | 16 | 1.6 |
| Pneumonitis | 8 | 0.78 |
| Respiratory failure | 4 | 0.39 |
| Aspiration | 3 | 0.29 |
| Adult respiratory distress syndrome | 1 | 0.1 |
| Organ failure/sepsis | 16 | 1.6 |
| Sepsis | 6 | 0.58 |
| Renal failure | 4 | 0.39 |
| Hypotension, shock | 3 | 0.29 |
| Multi organ system failure | 2 | 0.19 |
| Liver failure | 1 | 0.1 |
| Other | 20 | 1.9 |
| Hematoma | 6 | 0.58 |
| Vascular thrombosis | 6 | 0.58 |
| Seroma | 3 | 0.29 |
| Cholangitis | 2 | 0.19 |
| Pancreatitis | 2 | 0.19 |
| Catheter related infection | 1 | 0.1 |
| Death | 18 | 1.7 |
% of patients with that complication.
APPENDIX D. Correlations Coefficients for Adenocarcinoma Resections (n = 577)
| Estimated blood loss | Length of stay | Total fluids | Colloid | Crystalloid | Surgical time | |
|---|---|---|---|---|---|---|
| Estimated blood loss (ml) | 1.0 | 0.18 <0.0001 | 0.62 <0.0001 | 0.41 <0.0001 | 0.57 <0.0001 | 0.33 <0.0001 |
| Length of stay (days) | 0.18 <0.0001 | 1.0 | 0.18 <0.0001 | 0.15 0.0002 | 0.15 0.0002 | 0.27 <0.0001 |
| Total fluids (ml) | 0.62 <0.0001 | 0.18 <0.0001 | 1.0 | 0.52 <0.0001 | 0.97 <0.0001 | 0.57 <0.0001 |
| Colloid (ml) | 0.41 <0.0001 | 0.15 0.0002 | 0.52 <0.0001 | 1.0 | 0.31 <0.0001 | 0.31 <0.0001 |
| Crystalloid (ml) | 0.57 <0.0001 | 0.15 0.0002 | 0.97 <0.0001 | 0.31 <0.0001 | 1.0 | 0.55 <0.0001 |
| Surgical Time (min) | 0.33 <0.0001 | 0.27 <0.0001 | 0.57 <0.0001 | 0.31 <0.0001 | 0.55 <0.0001 | 1.0 |
APPENDIX E. Median Length of Stay Across Grades (% of Total Patients)
| Grade | 0 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| n | 592 (57%) | 118 (11%) | 111 (10%) | 183 (18%) | 7 (.06%) | 18 (1.7%) |
| LOS | 7 | 9 | 9 | 12 | 9 | 12 |
P < 0.001.
REFERENCES
- 1.Nisanevich V, Felsenstein I, Almogy G, et al. Effect of intraoperative fluid management on outcome after intraabdominal surgery. Anesthesiology. 2005;103:25–32. doi: 10.1097/00000542-200507000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Lindenblatt N, Park S, Alsfasser G, et al. Intraoperative fluid management in pancreatic resections—A surgeon’s view. Zentralblatt fur Chirurgie. 2008;133:168–175. doi: 10.1055/s-2008-1004745. [DOI] [PubMed] [Google Scholar]
- 3.Holte K, Klarskov B, Christensen DS, et al. Liberal versus restrictive fluid administration to improve recovery after laparoscopic cholecystectomy a randomized, double-blind study. Ann Surg. 2004;240:892–899. doi: 10.1097/01.sla.0000143269.96649.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holte K, Kristensen BB, Valentiner L, et al. Liberal versus restrictive fluid management in knee arthroplasty: A randomized, double-blind study. Anesth Analg. 2007;105:465–474. doi: 10.1213/01.ane.0000263268.08222.19. [DOI] [PubMed] [Google Scholar]
- 5.Brandstrup B, Tonnesen H, Beier-Holgersen R, et al. Effects of intravenous fluid restriction on postoperative complications: Comparison of two perioperative fluid regimens: A randomized assessor-blinded multicenter trial. Ann Surg. 2003;238:641–648. doi: 10.1097/01.sla.0000094387.50865.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holte K, Kehlet H. Fluid therapy and surgical outcomes in elective surgery: A need for reassessment in fast-track surgery. J Am College Surg. 2006;202:971–989. doi: 10.1016/j.jamcollsurg.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Neal JM, Wilcox RT, Allen HW, et al. Near-total esophagectomy: The influence of standardized multimodal management and intraoperative fluid restriction. Reg Anesth Pain Med. 2003;28:328–334. doi: 10.1016/s1098-7339(03)00197-4. [DOI] [PubMed] [Google Scholar]
- 8.Khoo CK, Vickery CJ, Forsyth N, et al. A prospective randomized controlled trial of multimodal perioperative management protocol in patients undergoing elective colorectal resection for cancer. Ann Surg. 2007;245:867–872. doi: 10.1097/01.sla.0000259219.08209.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lobo DN, Bostock KA, Neal KR, et al. Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: A randomised controlled trial. Lancet. 2002;359:1812–1818. doi: 10.1016/S0140-6736(02)08711-1. [DOI] [PubMed] [Google Scholar]
- 10.Walsh SR, Cook EJ, Bentley R, et al. Perioperative fluid management: Prospective audit. Int J Clin Pract. 2008;62:492–497. doi: 10.1111/j.1742-1241.2007.01386.x. [DOI] [PubMed] [Google Scholar]
- 11.MacKay G, Fearon K, McConnachie A, et al. Randomized clinical trial of the effect of postoperative intravenous fluid restriction on recovery after elective colorectal surgery. Br J Surg. 2006;93:1469–1474. doi: 10.1002/bjs.5593. [DOI] [PubMed] [Google Scholar]
- 12.DeOliveira ML, Winter JM, Schafer M, et al. Assessment of complications after pancreatic surgery: A novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg. 2006;244:931–937. doi: 10.1097/01.sla.0000246856.03918.9a. discussion 937–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grobmyer SR, Pieracci FM, Allen PJ, et al. Defining morbidity after pancreaticoduodenectomy: Use of a prospective complication grading system. J Am Coll Surg. 2007;204:356–364. doi: 10.1016/j.jamcollsurg.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Edis AJ, Kiernan PD, Taylor WF. Attempted curative resection of ductal carcinoma of the pancreas. Mayo Clin Proc. 1980;55:531–536. [PubMed] [Google Scholar]
- 15.Herter FP, Cooperman AM, Ahlborn TN, et al. Surgical experience with pancreatic and periampullary cancer. Ann Surg. 1982;195:274–281. doi: 10.1097/00000658-198203000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer M, Matsuo K, Gonen M, et al. Relationship between intraoperative fluid administration and perioperative outcome after pancreaticoduodenectomy: Results of a prospective randomized trial of acute normovolemic hemodilution compared with standard intraoperative management. Ann Surg. 2010;252:952–958. doi: 10.1097/SLA.0b013e3181ff36b1. [DOI] [PubMed] [Google Scholar]
- 17.Lavu H, Kennedy EP, Mazo R, et al. Preoperative mechanical bowel preparation does not offer a benefit for patients who undergo pancreaticoduodenectomy. Surgery. 2010;148:278–284. doi: 10.1016/j.surg.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Moore FD, editor. Metabolic care of the surgical patient. Philadelphia: W.B. Saunders Company; 1959. [Google Scholar]
- 19.Moore FD, Shires G. Moderation. Ann Surg. 1967;166:300–301. doi: 10.1097/00000658-196708000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell IT, Baxter JN, Tweedie IE, et al. IV fluids during surgery. Br J Anaesth. 1990;65:726–729. doi: 10.1093/bja/65.5.726. [DOI] [PubMed] [Google Scholar]
- 21.Jenkins MT, Giesecke AH, Johnson ER. The postoperative patient and his fluid and electrolyte requirements. Br J Anaesth. 1975;47:143–150. doi: 10.1093/bja/47.2.143. [DOI] [PubMed] [Google Scholar]
- 22.Rosenthal MH. Intraoperative fluid management—What and how much? Chest. 1999;115:106S–112S. doi: 10.1378/chest.115.suppl_2.106s. [DOI] [PubMed] [Google Scholar]
- 23.Shoemaker WWC, Appel PPL, Kram HHB, et al. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. 1988;94:1176–1186. doi: 10.1378/chest.94.6.1176. [DOI] [PubMed] [Google Scholar]
- 24.Shoemaker WWC, Appel PPL, Waxman KK, et al. Clinical trial of survivors’ cardiorespiratory patterns as therapeutic goals in critically ill postoperative patients. Crit Care Med. 1982;10:398–403. doi: 10.1097/00003246-198206000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Davidson GM. Intra-operative fluid and electrolyte requirements. Anesth Intensive Care. 1977;5:333–338. doi: 10.1177/0310057X7700500408. [DOI] [PubMed] [Google Scholar]
- 26.Grocott MPW, Mythen MG, Gan TJ. Perioperative fluid management and clinical outcomes in adults. Anesth Analg. 2005;100:1093–1106. doi: 10.1213/01.ANE.0000148691.33690.AC. [DOI] [PubMed] [Google Scholar]
- 27.Prien T, Backhaus N, Pelster F, et al. Effect of intraoperative fluid administration and colloid osmotic pressure on the formation of intestinal edema during gastrointestinal surgery. J Clin Anesth. 1990;2:317–322. doi: 10.1016/0952-8180(90)90077-g. [DOI] [PubMed] [Google Scholar]
- 28.Mythen MG, Webb AR. Intra-operative gut mucosal hypoperfusion is associated with increased post-operative complications and cost. Intensive Care Med. 1994;20:99–104. doi: 10.1007/BF01707662. [DOI] [PubMed] [Google Scholar]
- 29.Twigley AJ, Hillman KM. The end of the crystalloid era? Anaesthesia. 1985;40:860–871. doi: 10.1111/j.1365-2044.1985.tb11047.x. [DOI] [PubMed] [Google Scholar]
- 30.Holte K, Jensen P, Kehlet H. Physiologic effects of intravenous fluid administration in healthy volunteers. Anesth Analg. 2003;96:1504–1509. doi: 10.1213/01.ANE.0000055820.56129.EE. [DOI] [PubMed] [Google Scholar]
- 31.Holte K, Sharrock NE, Kehlet H. Pathophysiology and clinical implications of perioperative fluid excess. Br J Anaesth. 2002;89:622–632. doi: 10.1093/bja/aef220. [DOI] [PubMed] [Google Scholar]
- 32.Lang K, Boldt J, Suttner S, et al. Colloids versus crystalloids and tissue oxygen tension in patients undergoing major abdominal surgery. Anesth Analg. 2001;93:405–409. doi: 10.1097/00000539-200108000-00034. [DOI] [PubMed] [Google Scholar]
- 33.Arieff AIM. Fatal postoperative pulmonary edema. Chest. 1999;115:1371–1377. doi: 10.1378/chest.115.5.1371. [DOI] [PubMed] [Google Scholar]
- 34.Desborough JP. The stress response to trauma and surgery. Br J Anaesth. 2000;85:109–117. doi: 10.1093/bja/85.1.109. [DOI] [PubMed] [Google Scholar]
- 35.Brandstrup B. Fluid therapy for the surgical patient. Best Pract Res Clin Anaesthesiol. 2005;20:265–283. doi: 10.1016/j.bpa.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Futier E, Constantin JM, Petit A, et al. Conservative vs restrictive individualized goal-directed fluid replacement strategy in major abdominal surgery: A prospective randomized trial. Arch Surg. 2010;145:1193–1200. doi: 10.1001/archsurg.2010.275. [DOI] [PubMed] [Google Scholar]
- 37.Brandstrup B, Engquist A. Is postoperative mortality affected by liberal intravenous fluid therapy? Presentation of a Cochrane analysis and a literature review. Ugeskr Laeger. 2003;165:1342–1345. [PubMed] [Google Scholar]
- 38.de Aguilar-Nascimento J, Diniz B, do Carmo AV, et al. Clinical benefits after the implementation of a protocol of restricted perioperative intravenous crystalloid fluids in major abdominal operations. World J Surg. 2009;33:925–930. doi: 10.1007/s00268-009-9944-2. [DOI] [PubMed] [Google Scholar]
- 39.Muller S, Zalunardo MP, Hubner M, et al. A fast-track program reduces complications and length of hospital stay after open colonic surgery. Gastroenterology. 2009;136:842–847. doi: 10.1053/j.gastro.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 40.Vermeulen H, Hofland J, Legemate DA, et al. Intravenous fluid restriction after major abdominal surgery: A randomized blinded clinical trial. Trials. 2009;10:50. doi: 10.1186/1745-6215-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]