FIGURE 3.
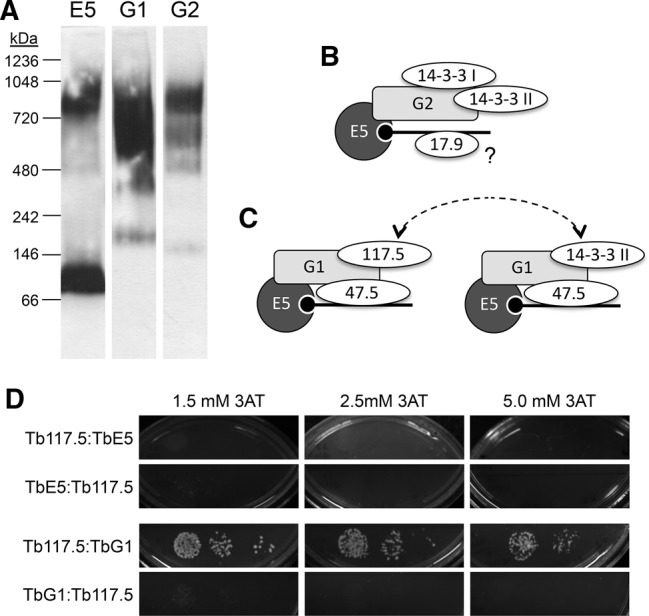
TbEIF4E5 is present in high molecular weight complexes. (A) Blue Native gel electrophoresis of cell extracts from transfected T. brucei containing PTP-tagged proteins. Lysates were run through Blue Native gels, transferred to nitrocellulose membranes and probed with antibody directed against the protein A domain of the PTP tag (TbE5, TbG1, TbG2 = E5, G1, G2, respectively). The migration of size standards is indicated in kDa. (B) The components that co-purified with TbEIF4E5 and TbEIF4G2. The RNA (black line; black circle represents 5′ end cap 4) is recognized by the cap-binding eIF4E component TbE5 (dark gray circle, E5). The eIF4G-like scaffold protein TbG2 (light gray rectangle, G2) interacts directly with TbE5, as indicated by overlap of the respective shapes. Other TbE5-associated proteins are shown as ovals. Overlap of the ovals with other shapes is speculative and based on the assumed scaffold function of TbG2, and shapes do not reflect protein size. The placement of Tb17.9 on the RNA is speculative, as indicated by the “?”. (C) The TbEIF4E5/TbEIF4G1 dynamic complex model. The 117.5-kDa protein in association with the TbG1 scaffold may be regulated by the presence of the 14-3-3 II homodimer, the binding of which is dependent on TbG1 phosphorylation. The dotted arrows highlight the either/or aspect of the complexes. (D) Yeast two-hybrid analysis of Tb117.5 with TbE5 or TbG1 in both bait and prey orientations. Controls and interpretations are shown in Figure 2.
