Abstract
Autosomal recessive cutis laxa type I (ARCL type I) is characterized by generalized cutis laxa with pulmonary emphysema and/or vascular complications. Rarely, mutations can be identified in FBLN4 or FBLN5. Recently, LTBP4 mutations have been implicated in a similar phenotype. Studying FBLN4, FBLN5 and LTBP4 in 12 families with ARCL type I, we found bi-allelic FBLN5 mutations in 2 probands, whereas 9 probands harbored biallelic mutations in LTBP4. FBLN5 and LTBP4 mutations cause a very similar phenotype associated with severe pulmonary emphysema, in the absence of vascular tortuosity or aneurysms. Gastro-intestinal and genitourinary tract involvement seems to be more severe in patients with LTBP4 mutations. Functional studies showed that most premature termination mutations in LTBP4 result in severely reduced mRNA and protein levels. This correlated with increased transforming growth factor beta (TGFβ) signaling. However, one mutation, c.4127dupC, escaped nonsense-mediated decay. The corresponding mutant protein (p.Arg1377Alafs*27) showed reduced colocalization with fibronectin, leading to an abnormal morphology of microfibrils in fibroblast cultures, while retaining normal TGFβ signaling. We conclude that LTBP4 mutations cause disease through both loss of function and gain of function mechanisms.
Keywords: LTBP4, FBLN5, Urban-Rifkin-Davis syndrome, fibrillin, cutis laxa, recessive
INTRODUCTION
Cutis laxa refers to a heterogeneous group of rare connective tissue disorders, characterized by the presence of loose, sagging and inelastic skin that may present with systemic manifestations of variable severity. Both hereditary and acquired forms exist. Acquired forms appear secondary to infections, administration of medications or as a paraneoplasm (Lewis, et al., 2004). Historically, classification of the hereditary forms is based on clinical grounds and mode of inheritance. Autosomal dominant, recessive and X-linked forms have been described (de Schepper, et al., 2003). The X-linked recessive form, known as occipital horn syndrome (MIM# 304150), comprises skeletal manifestations including the pathognomonic occipital horns and bladder diverticula. Patients carry mutations in the ATP7A gene encoding a copper transporter resulting in low serum copper and ceruloplasmin concentrations (Kaler, et al., 1994). Autosomal dominant cutis laxa (ADCL; MIM# 123700) was historically considered a benign disease confined to the skin, but severe emphysema and aortic root dilatation was recently described in several patients (Callewaert, et al., 2011; Szabo, et al., 2006; Urban, et al., 2005). Heterozygous mutations in both the elastin (ELN; MIM# 130160) and fibulin 5 (FBLN5; MIM# 604580) genes have been reported in ADCL (Callewaert, et al., 2011; Markova, et al., 2003; Szabo, et al., 2006; Tassabehji, et al., 1998; Urban, et al., 2005). Autosomal recessive cutis laxa (ARCL) comprises several subtypes. ARCL type I associates generalized cutis laxa with life-threatening pulmonary and/or arterial involvement. Patients with homozygous FBLN5 mutations (autosomal recessive cutis laxa type Ib) encounter pulmonary emphysema as the most prominent non-dermatological feature (Loeys, et al., 2002), while mutations in the gene encoding fibulin-4 (FBLN4/EFEMP2; MIM# 604633) cause severe tortuosity, aneurysm formation, stenoses, and usually less prominent cutis laxa (Hucthagowder, et al., 2006; Renard, et al., 2010) (autosomal recessive cutis laxa type Ia). The majority of cases, however, remain molecularly unexplained. ARCL type II (MIM# 219200) is characterized by distinct facial features, skeletal abnormalities and variable neurological involvement. Mutations in ATP6V0A2, encoding an ATPase proton pump subunit, and PYCR1, which encodes the mitochondrial pyrroline-5-carboxylate reductase 1, are implicated in ARCL type II (Kornak, et al., 2008; Reversade, et al., 2009). In type III ARCL, also known as De Barsy syndrome, clinical hallmarks are mental retardation, athetosis and corneal clouding. In some of these patients, ATP6V0A2, PYCR1, or ALDH18A1 have been identified (Bicknell, et al., 2008; Guernsey, et al., 2009; Leao-Teles, et al., 2009).
Recently, mutations in the gene encoding the latent transforming growth factor beta binding protein 4 (LTBP4; MIM# 604710) were identified in patients with cutis laxa and a constellation of impaired pulmonary, gastro-intestinal, genitourinary, musculoskeletal and dermal development, named Urban-Rifkin-Davis syndrome (MIM# 613177) (Urban, et al., 2009) and more recently designated as autosomal recessive cutis laxa type Ic. Patients present with generalized cutis laxa, emphysema and genitourinary and gastro-intestinal diverticula. To date, only 5 LTBP4 mutations have been reported in a total of 4 patients. LTBP4 encodes a member of the group of latent transforming growth factor beta binding proteins (LTBPs) that take part in the formation of the microfibrillar structures. Transforming growth factor-beta (TGFβ) is secreted into the extracellular matrix as a small latent complex (SLC), bound to its latency associated peptide. This SLC, in turn, binds to some of the LTBPs, including the long form of LTBP4, to form a large latent complex (LLC). As such, TGFβ molecules are sequestered into the microfibrillar structures, from which release is controlled through various mechanisms (Annes, et al., 2004; de Cavanagh, et al., 2009; Jobling, et al., 2006; Koli, et al., 2008; Wipff and Hinz, 2008; Wipff, et al., 2007). In this context, LTBP4 plays an important role in the regulation of TGFβ1 signaling.
Considerable clinical overlap exists among the different entities within type I recessive cutis laxa. In this paper we analyzed the FBLN4, FBLN5 and LTBP4 in 12 probands presenting with ARCL and pulmonary emphysema and/or bladder diverticula. Our results help to differentiate among phenotypes and to direct molecular analyses. In addition, functional investigations of the consequences of LTBP4 mutations provide new insights into the molecular mechanisms of this type of cutis laxa.
MATERIAL AND METHODS
Patient population
We clinically and molecularly characterized a series of 12 probands (20 patients) with ARCL and pulmonary emphysema (family A-K) and/or bladder diverticula with normal serum copper and ceruloplasmin (Family L). None of the patients in this study has been reported previously. All patients were clinically evaluated by an experienced clinical geneticist. Cardiovascular imaging was done by means of an echocardiography by an experienced children’s cardiologist in all patients. CT-thorax (for pulmonary assessment) was performed in C:II-2, D:IV-2, G:II-1, I:IV-6. In addition, cardiovascular catheterization was performed in patient J:II-1 and in patient I:IV-6 vessels were directly inspected during abdominal surgery. All patients or a legal representative consented to the study. Special informed consents were obtained for the publication of clinical pictures. The study was approved by Ethics Committee of the Ghent University Hospital and the University of Pittsburgh Institutional Review Board.
Molecular analysis of FBLN4, FBLN5 and LTBP4
Genomic DNA from patients and available family members was extracted from peripheral blood samples using the Puregene method (Qiagen – Valencia, CA) or dermal fibroblast cultures (DNeasy - Qiagen – Valencia, CA) for the probands of family C, E, I. For patient F.II.1, no samples were available for formal testing, and DNA samples from both parents were used for diagnostic analysis instead.
Specific primers were designed for all exons with flanking intronic sequences of FBLN4, FBLN5, and LTBP4 (primer sequences available on request). Sanger sequencing of purified PCR amplicons was performed on an ABI 3730 automatic sequencer using Big Dye Termination reaction version 3.1 (Applied Biosystems, Halle, Belgium). Obtained sequence profiles were compared with the FBLN4 (NM_016938.3), FBLN5 (NM_006329.3) and the LTBP4 (NM_003573.2) reference sequences using the Seqscape software package (Applied Biosystems, Foster City, CA, USA). For cDNA numbering, +1 corresponds to the A of the ATG translation initiation codon. For protein numbering, +1 corresponds to the first translated methionine.
Mutations were confirmed to segregate according to disease status in families where DNA samples of parents and/or siblings were available. Missense mutations were designated deleterious according to SIFT and Polyphen mutation prediction programs. None of the mutations reported here were known as a single nucleotide polymorphism in dbSNP or Ensembl. All mutations were submitted to the Leiden Open Variation Database at URL http://www.LOVD.nl/FBLN5 and http://www.LOVD.nl/LTBP4.
qPCR analysis of LTBP4 expression levels
Total RNA was extracted from dermal fibroblasts of patients of family C, E and I using the RNeasy method (Qiagen – Valencia, CA) according to the manufacturer’s protocol. Triplicate cultures were extracted for each patient. Complementary DNA (cDNA) was obtained using the iScript reverse transcriptase reaction (Bio-Rad, Hercules, CA). qPCR primers were designed for LTBP4 and 3 reference genes (YWHAZ, GAPDH and HPRT1). Assays were run in triplicate for each sample on a Roche Lightcycler 480 system using Realtime Ready DNA probes Mastermix supplemented with ResoLight Dye (Roche) according to the manufacturer’s protocol.
Immunoblotting
Media samples were collected with a 1:200 dilution of protease inhibitor cocktail (Sigma) after treating the cultured skin fibroblasts overnight in serum free media. Amicon Ultra-15 centrifugal filter units (Millipore) were used to concentrate media samples. Proteins (20µg) were separated by 5% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto polyvinylidene difluoride membrane (Millipore). Non-specific binding sites were blocked by incubation in PBS-0.2% Tween20 containing 10% dried skim milk at 4ºC overnight. Blotted membranes were probed with antibodies against LTBP4 or fibrillin-1 (Supp. Table S1) at room temperature for one hour, followed by incubation with HRP-conjugated donkey anti-goat secondary antibody (Santa Cruz) or HRP-conjugated goat anti-rabbit secondary antibody (Thermo Scientific). Immunoreactive bands were detected using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific).
Immunofluorescent Staining
Skin fibroblasts were plated on 22×22-mm square glass coverslips (Fisher Scientific) in 6-well tissue culture dishes at a density of 3000 cells/cm2 and cultured in 2 mL Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine (FBS). Cells were stained 4 weeks after confluency. Briefly, cells were rinsed with PBS, fixed with PBS containing 4% paraformaldehyde (Electron Microscopy Sciences) at room temperature for 15 minutes and rinsed three times with PBS for 5 minutes. Incubation overnight in 3% BSA with 20% donkey serum and 0.3 M glycine to avoid background and non-specific staining was performed. The coverslips were incubated with anti-LTBP4, anti-fibrillin-1, mouse anti-LTBP1, anti-fibulin-5, and anti-fibronectin primary antibodies ( Supp. Table S1) at room temperature for 1 hour and were then washed three times with PBS at 5-minute intervals. Secondary antibodies were donkey anti-rabbit Alexa 488 (Invitrogen), donkey anti-mouse Alexa 594 (Invitrogen) as well as donkey anti-goat Alexa 594 (Invitrogen) and the incubation time for secondary antibodies was 1 hour at room temperature. After counterstaining with Hoechst 33258 (Sigma), cover slips were rinsed again three times with PBS and mounted with Cytoseal-60 mounting media (Thermo Scientific) after being completely dried. Specimens were examined and photographed using a fluorescence photomicroscope (Leica DM5000B, Leica Microsystems).
Luciferase Assay for latent TGFβ Activity
Cultured reporter mink lung epithelial cells (MLECs) (Abe, et al., 1994), stably transfected with an expression construct containing a plasminogen activator inhibitor-1 promoter fused to the firefly luciferase gene, were used to detect the activity of transforming growth factor beta (TGFβ) in human fibroblasts by Luciferase Assay System (Promega, catalog NO. E4550). Co-cultured MLECs and fibroblasts cells of controls and patients were each suspended at 2 × 105 /ml in DMEM containing 2.0% FBS. MLECs were plated at 100µl per well in 96-well plates, following by fibroblasts cells being added in the same cell numbers to each well. A standard curve was constructed with decreasing TGFβ1 (PeproTech; Recombinant Human TGFβ1) concentration from 2000 pg/ml in MLECs suspension and performed 2:1 dilution by MLECs suspension successively. Cells were incubated for 16 hours. Cell lysates were prepared in Reporter Lysis Buffer (Promega, catalog No. E3971) and assayed for luciferase activity by Luciferase Assay System. Luminescence units were converted to TGFβ1 concentration with the use of the standard curve.
Electron microscopy
Skin biopsies were fixed in glutaraldehyde, stained sequentially with OsO4, tannic acid, and uranyl acetate, dehydrated, and embedded in Epon (Davis, 1993). Thin sections (60 nm) were cut, placed on formvar coated grids, and counterstained with 7% methanolic uranyl acetate and lead citrate. Sections were viewed with a Tecnai 12 transmission electron microscope at 120 kV, and the images were digitally captured.
RESULTS
Clinical characteristics of the selected cohort
The clinical characteristics of the studied patients are presented in Supp. Table S2. Pedigrees are shown in Supp. Figure S1. Probands originated from the Middle-East (4/12), Turkey (2/12), North-Africa (1/12), Europe (4/12), or the USA (1/12). Consanguinity was reported for 10 probands, a family history for cutis laxa was present in 6. Female to male ratio was 8/4 in the probands, 12/7 when family members were included. Mean age at last follow-up was 4.8 years for the probands. All patients presented with moderate to severe cutis laxa with variable involvement of the facial skin. Except for proband L:II-1, all had pulmonary involvement of varying degree. In the cardiovascular system, we found peripheral pulmonary artery stenosis (PPAS) in 6 probands, and valvular involvement in 6. Mild to moderate regurgitation of the mitral (G:II-1), tricuspid (G:II-1, J:II-1, K:IV-1), aortic (B:IV-2, G:II-1, I:IV-6) and pulmonary valves (G:II-1) occurred due to floppy (G:II-1, J:II-1, K:IV-1) and/or dysplastic valves (B:IV-2, G:II-1, I:IV-6). The dysplastic pulmonary and aortic valves in patient I:IV-6 also showed moderate stenosis. Patient L:II-1 only had mitral valve stenosis. No arterial tortuosity or aneurysms could be documented upon evaluation with echocardiography, CT-thorax (C:II-2, D:IV-2, G:II-1, I:IV-6), cardiovascular catheterization (J:II-1) or upon inspection during surgery (I:IV-6). Gastro-intestinal diverticula were present in one proband and resulted in a bowel perforation. Rectal prolapse occurred in 2 probands. Five probands had bladder diverticula. However, due to the critical illness of many patients upon admission, no extensive imaging of the vascular, gastro-intestinal and genito-urinary system has been routinely performed in the absence of symptoms. Muscular hypotonia was noted in 5 probands, but all patients had a normal mental status. Nine out of 12 probands died before the age of 14 years old. The cause of death was related to pulmonary disease in eight probands.
Mutational analysis
Molecular analysis of FBLN4 in our patient cohort did not reveal any mutations. Sequencing of the FBLN5 gene identified 2 mutations in two families: a homozygous c.1171G>T (p.Glu391X) alteration in family A and a homozygous c.649T>C (p.Cys217Arg) mutation in family B (Table 1, Figure 1A). The latter had previously been reported by Claus et al., in a Lebanese family (Claus, et al., 2008). This missense substitution alters the second cysteine of the 4th cbEGF-like domain, which forms a disulfide bond with a cysteine at position 230. These residues are highly conserved and important for the linear stabilization of the molecule.
Table 1.
Mutations in the FBLN5 and LTBP4 gene identified in this study
| Family | Gene | cDNA | Protein | Type | Domain |
|---|---|---|---|---|---|
| Family A | FBLN5 | c.1171G>T | p.Glu391X | Nonsense | FBLN specific domain |
| Family B | FBLN5 | c.649T>C | p.Cys217Arg | Missense | 4th cbEGF-like domain |
| Family C | LTBP4 | c.1342C>T | p.Arg448X | Nonsense | 1st 8-Cys domain |
| c.4115dupC | p.Tyr1372Ilefs*2 | Frameshift - PTC | 3rd 8-Cys domain | ||
| Family D | LTBP4 | c.2408C>A | p.Ser803X | Nonsense | 7th EGF-like domain |
| Family E | LTBP4 | c.3661C>T | p.Gln1221X | Nonsense | 2nd 8-Cys domain |
| c.3886C>T | p.Gln1296X | Nonsense | 14th EGF-like domain | ||
| Family F | LTBP4 | c.780+2T>G | NA | Splicesite | - |
| Family G | LTBP4 | c.1263delC | p.Cys422Alafs*352 | Frameshift - PTC | 1st 8-Cys domain |
| Family H | LTBP4 | c.1851C>A | p.Cys617X | Nonsense | 2nd EGF-like domain |
| Family I | LTBP4 | c.4127dupC | p.Arg1377Alafs*27 | Frameshift - PTC | 3rd 8-Cys domain |
| Family J | LTBP4 | c.4129C>T | p.Arg1377X | Nonsense | 3rd 8-Cys domain |
| Family K | LTBP4 | c.3556T>C | p.Cys1186Arg | Missense | 2nd 8-Cys domain |
Mutation nomenclature refers to the FBLN5 (NM_006329.3) and LTBP4 (NM_003573.2) reference sequences. For cDNA numbering, +1 corresponds to the A of the ATG translation initiation codon. For protein numbering, +1 corresponds to the first translated methionine. PTC, premature truncation; EGF-like, epidermal growth factor-like; cb, calcium binding.
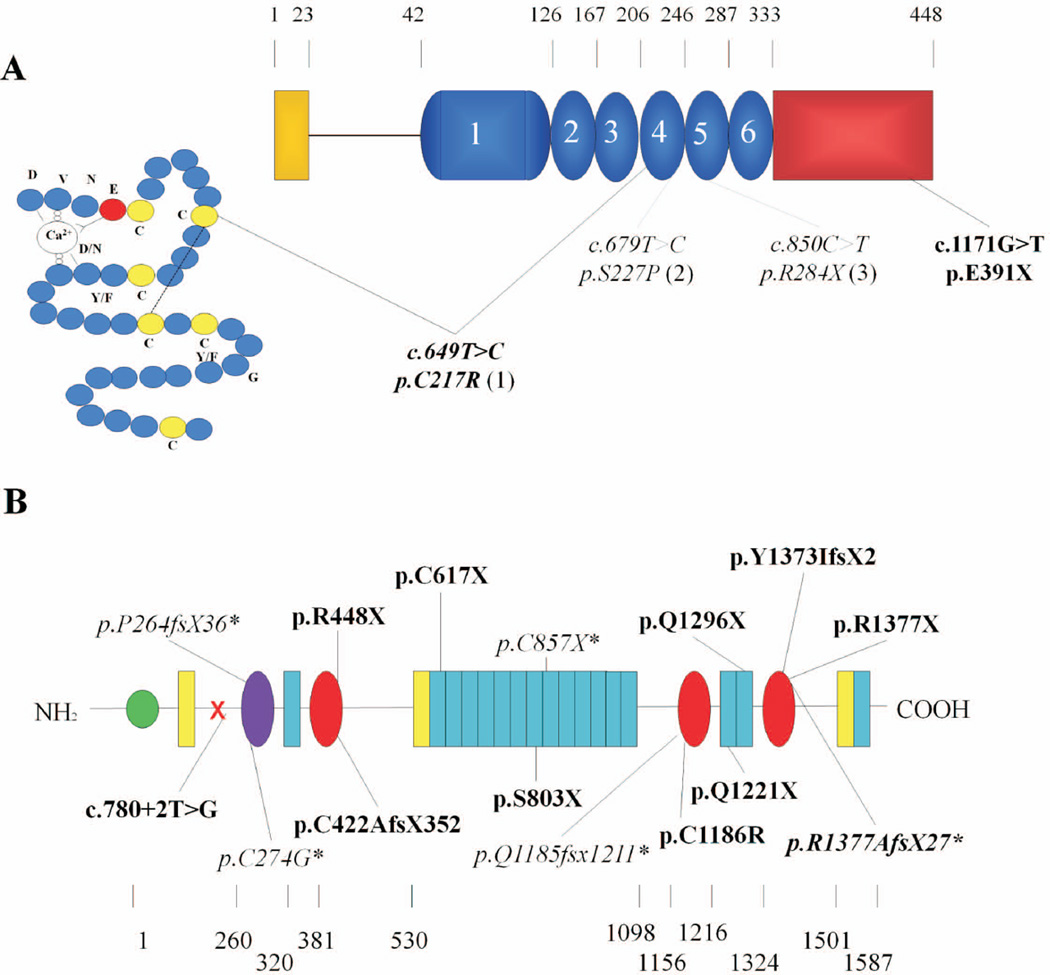
Figure 1. Schematic representation of Fibulin-5 (panel A) and LTBP4 (panel B), with all identified mutations to date.
An amino acid scale is shown above and below the schematic representation of the fibulin-5 and LTBP4 proteins, respectively. Mutations identified in this study are depicted in bold. Previously reported mutations are shown in italic and marked with (1) (Claus, et al., 2008); (2) (Elahi, et al., 2006; Loeys, et al., 2002); (3) (Nascimento, et al., 2010); or * (Urban, et al., 2009). A: Fibulin-5. Yellow, Signal peptide, Blue, calcium binding epidermal growth factor – like domain (cbEGF-like) (the first cbEGF-like domain includes an insertion); Red, Fibulin specific globular domain. The 4th EGF-like domain is shown in detail. The disulfide bond between C217 and C230 is indicated by a dashed line. B: The long form of LTBP4. Green, 4-Cys domain; red, 8-Cys (TB) domain; purple, hybrid domain; blue, calcium binding epidermal growth factor-like domain; yellow, EGF-like domain.
Subsequent sequencing of the LTBP4 gene identified 10 novel and one previously reported mutation (c.4127dupC) (Urban, et al., 2009) in 9 families (Table 1, Figure 1B). Six mutations introduce a nonsense codon: (p.Arg448X), (p.Ser803X), (p.Gln1221X), (p.Gln1296X), (p.Cys617X), (p.Arg1377X). Three mutations were single base pair deletions or insertions resulting in a frameshift and a downstream premature termination codon (PTC): c.4115dupC (p.Tyr1373Ilefs*2), c.1263delC (p.Cys422Alafs*352), c.4127dupC (p.Arg1377Alafs*27). The c.4127dupC mutation is located in the polyC homopolymer tract in exon 33.
We further identified a homozygous p.Cys1186Arg missense mutation in family K that affects a highly conserved cysteine residue within the 2nd 8-Cys domain of LTBP4 (Figure 1B) and is predicted to be damaging by Polyphen and SIFT algorithms. Finally, a splice site mutation c.780+2T>G in intron 8 (family F), disrupts the conserved splice donor site sequence and impairs normal splicing as supported by in silico splice site analysis using BDGP splice site prediction software (http://www.fruitfly.org/seq_tools/splice.html). The exact effect of this mutation on mRNA splicing could not be examined due to unavailability of patient fibroblasts. The remaining patient, negative for FBLN4, FBLN5 and LTBP4 mutations, was also screened for the ELN gene, but no mutations were identified.
Clinical characteristics of the patients with FBLN5 mutations
The clinical details are summarized in the Supp. Table S2 and illustrated in Supp. Figure S2A.
The proposita of family A was the fifth child of a consanguineous (inbreeding coefficient 1/8) Algerian union. The family history was remarkable for two spontaneous abortions around a gestational age of 3 months. Two older sisters had congenital generalized cutis laxa and died early. The proband had severe congenital generalized cutis laxa with facial involvement. At 19 months of age, she presented with a high and broad forehead, a low and broad nasal bridge, a beaked nose, large dysplastic ears, sagging cheeks and an everted lower lip (Figure 2A). She had severe emphysema and a normal cardiac status on echocardiography. She had photophobia and hypotonia, but a normal mental status.
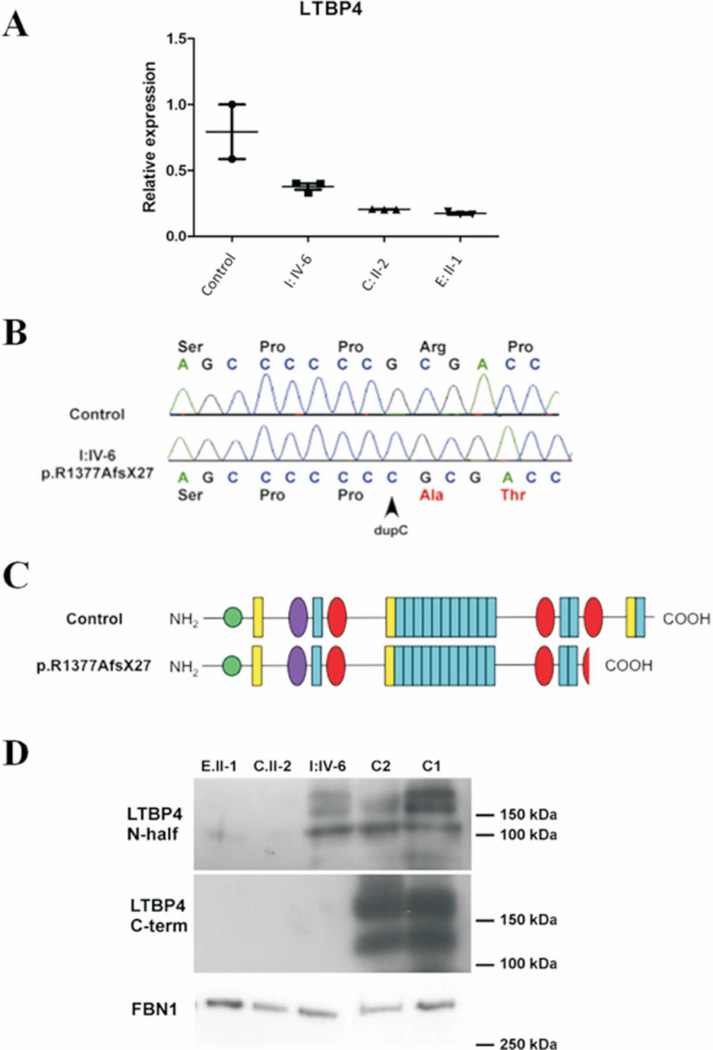
Figure 2. Expression analysis of LTBP4 at the mRNA and protein levels.
A: Relative expression of LTBP4 mRNA (normalized to expression of household genes YWHAZ, GAPDH, and HPRT1) on total RNA samples extracted from patient skin fibroblasts (I:IV-6, C:II-2, E:II-1). Premature termination mutations resulted in greatly reduced levels of LTBP4 mRNA in C:II-1, E:II-1, and a more modest reduction in I:IV-6. Bars indicate 95% confidence interval. B: Sequencing of a RT-PCR product from fibroblast I:IV-6 shows the presence of mutation c. 4127dupC (p.Arg1377Alafs*27) compared to a control. C: Schematic representation of the truncated LTBP4 protein predicted based on the presence of the mutation p.Arg1377Alafs*27 compared to the full length (long form) of LTBP4 protein. D: Representative image of immunoblots of LTBP4 and fibrillin-1 (FBN1) in conditioned media from control and mutant fibroblasts. LTBP4 was probed using an antibody raised against the amino-terminal half of LTBP4 (N-half) and an antibody against the carboxy-terminus (C-term). LTBP4 is detected as a long form (~175kDa), a short form (~150 kD) and a proteolytic fragment (~120kDa). Fibrillin-1 appears as a single 350 kD protein. C1: control 1; C2: control 2. Patients: C:II-2, E:II-1 and I:IV-6. Severely reduced expression of LTBP4 was noted in patient C:II-2 and E:II-1. LTBP4 was detectable in the media of I:IV-6 using the anti-N-half antibody but not using the anti-C-term antibody.
Family B originated from Lebanon. The consanguineous couple (inbreeding coefficient 1/8) had three affected children with severe congenital generalized cutis laxa. There were no unaffected children. The 9 year old boy had severe generalized congenital cutis laxa, inguinal hernias, mild aortic valve regurgitation and stenosis. The second child (female proband) died at 11 months following a bronchiolitis in a wider context of obstructive lung disease. She also had inguinal hernias, peripheral pulmonary artery stenosis and mild aortic and tricuspid regurgitation. The third child was a 2 year old boy with aortic insufficiency and peripheral pulmonary artery stenosis. Remarkably, all children had pyloric stenosis.
Clinical characteristics of the patients with LTBP4 mutations
The clinical details are summarized in the Supp. Table S2 and illustrated in Supp. Figure S2.
Most LTBP4 mutation positive patients (11/13) had generalized moderate to severe cutis laxa (Supp. Figure S2A). In patient C:II-2 skin manifestations mainly localized to thorax, abdomen and thighs. Patient J:II-1 had mild cutis laxa with mainly a hyperextensible skin. Involvement of the facial skin, resulting in a coarse and aged appearance was present in most probands (8/9), but varied in severity. In some individuals the skin was hyperextensible, or appeared translucent with a prominent venous pattern (3/9) (D:IV-1, E:II-1 and G:II-1). A few patients had thin and slowly growing hair. Inguinal and diaphragmatic (Supp. Figure S2B) hernias (5/9) were frequent and the latter often required surgical correction.
Pulmonary involvement with emphysema was universally present. In the majority of cases the emphysematous changes were severe. Upper airway involvement with tracheomalacia (patient K:IV-1) aggravated respiratory symptoms. Only in patient J:II-1 emphysema was mild upon CT-evaluation, but her lung function tests showed severe obstructive lung disease with a Tiffeneau index (FEV1/FVC) of 41 % (50% after bronchodilation).
In the majority of patients, cardiovascular involvement was limited to peripheral pulmonary artery stenosis (5/9 probands) that did not require any intervention although ventilation/perfusion mismatch may worsen oxygenation due to emphysematous lung changes. Pulmonary hypertension and peripheral pulmonary artery stenosis can develop secondary to emphysema (Matsuoka, et al.), a likely scenario in these patients. We did not observe any patients with supravalvular aortic stenosis. Both valvular regurgitation due to floppy and/or dysplastic valves (4/9) and stenosis due to dysplastic aortic and pulmonary valves (1/9) occurred. One patient was reported with a non-obstructive atrial septum aneurysm. Life-threatening pulmonary hypertension was observed in 4 patients (H:IV-1, I:IV-6, J:II-1, K:IV-1) complicating artificial ventilation.
Bladder diverticula (Supp. Figure S2B) occurred in more than half of all patients and caused inadequate voiding with urinary tract infections. Proband C needed an artificial bladder. Secondary hydronephrosis was seen in patient D:IV-2, K:IV-1 and J:II-1, requiring an ureterostomy in the latter. Fragility of the gastrointestinal tissues was demonstrated by diverticula and rectal prolapse. Remarkably, in family I all 3 affected sibs suffered from gastric or bowel ruptures. Lengthening of the gastrointestinal tract resulted in tortuosity in patient I:IV-6.
The neurological status of all patients seemed within normal range, although assessment was complicated by the fact that many patients were very young and critically ill at last evaluation. Hypotonia (4/9) and joint laxity (2/9) were observed. Craniofacial dysmorphism included sloping forehead, sparse hair on the temporal sides, large ears, hypertelorism, a low nasal bridge, a beaked nose, sagging cheeks, and retrognathia (Supp. Figure S2A). In family K, Kartagener syndrome with primary ciliary dyskinesia and situs inversus totalis segregated independently of ARCL, since this was also seen in a family member without cutis laxa features.
Overall, the prognosis was poor with a mortality rate over 80% (8/9 probands, 14/17 patients). Mean age at death of the probands was around 4 years (median 6 months, range 4 weeks to 13 years). Most patients succumbed to respiratory failure, often triggered by infectious episodes or in a perioperative setting. Two patients of a same family did not survive a perforation of the gastrointestinal tract. One patient died of unexplained brain abscesses. Three spontaneous abortions were reported in family I, but no material was preserved to assess the genetic status of these fetuses.
Expression of LTBP4 in patient fibroblasts
QPCR analysis for LTBP4 mRNA in available skin fibroblasts of patients C:II-2 (p.Arg448X/p.Tyr1373Ilefs*2,), E:II-1 (p.Gln1221X/p.Gln1296X,) and I:IV-6 (p.Arg1377Alafs*27, homozygous) showed diminished LTBP4 transcript levels in all 3 patients, indicating nonsense mediated decay (NMD) in case of premature termination mutations (Figure 2A). However, I:IV-6 showed residual expression of mutant LTBP4 mRNA, suggesting partial escape from NMD. Sequencing of RT-PCR products confirmed that the LTBP4 mRNA in fibroblasts form I:IV-6 contained the frameshift mutation p.Arg1377Alafs*27 (Figure 2B). This mutation was predicted to cause a truncation of LTBP4 within the 3rd TGFβ-binding (TB) domain (Figure 2C).
To test if the LTBP4 mutations affected expression at the protein level too, conditioned media samples from control and mutant fibroblasts were analyzed by immunoblotting (Figure 2D). LTBP4 could not be detected in C.II-2 and E.II-1, but was detectable in I:IV-6 using an antibody raised against the amino-terminal half of LTBP4 (LTBP4 N-half). This finding is consistent with our qPCR data (Figure 2A). Conversely, an antibody raised against the carboxy-terminus of LTBP4 (LTBP4 C-term) failed to detect any LTBP4 in fibroblasts from I:IV-6. Thus, immunoblotting confirmed that mutation p.Arg1377Alafs*27 in patient I:IV-6 partially escaped NMD resulting in a C-terminally truncated protein secreted at comparable levels to control fibroblasts. Fibrillin-1 expression in conditioned media did not show significant difference between patients and controls (Figure 2D, FBN1).
Altered assembly of elastic fibers in LTBP4 mutant fibroblasts and tissue samples
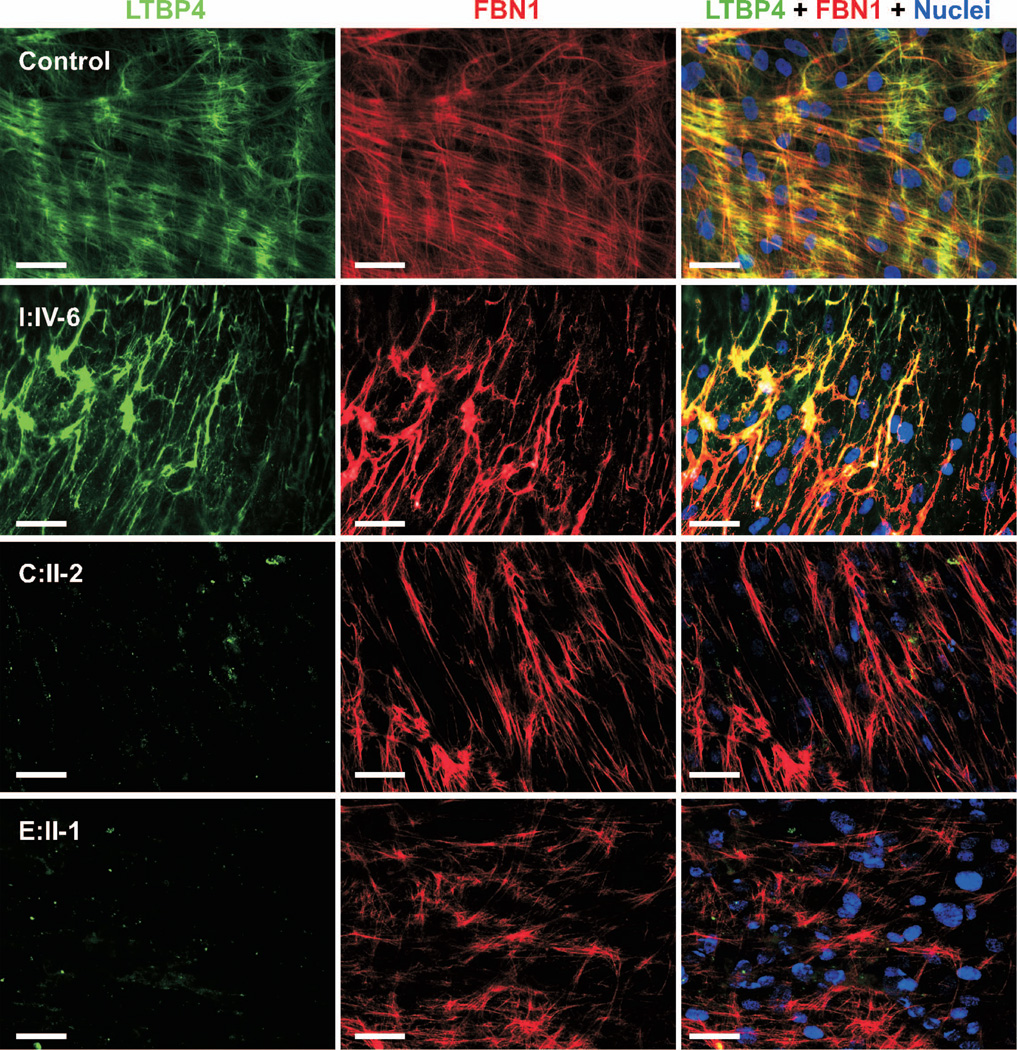
To investigate if loss of LTBP4 expression in patient fibroblasts affected elastic fiber assembly, we performed double immunostaining for LTBP4 and fibrillin-1 (Figure 3). Five controls were examined with one representative control image shown in Figure 3. LTBP4 in control fibroblasts was localized to thin and straight extracellular matrix fibrils exhibiting patchy overlap with fibrillin-1. Consistent with our qPCR and immunoblotting results, fibroblasts from patients C.II-2 and E.II-1 had no extracellular LTBP4 staining, but fibrillin-1 microfibrils were normal. Interestingly, LTBP4 in patient I:IV-6 fibroblasts showed strong extracellular matrix staining and complete colocalization with fibrillin-1 microfibrils, which were abnormally thick and wavy instead of thin and straight. Thus, it appeared that the truncated LTBP4 altered fibrillin-1 microfibril bundle formation and elongation.
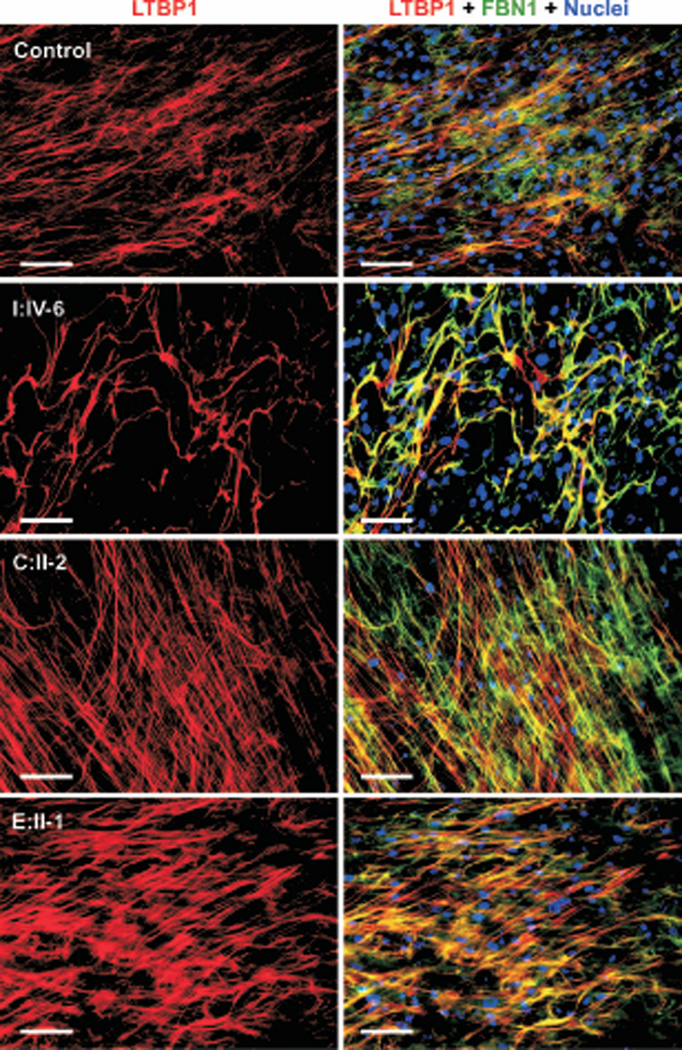
Figure 3. Immunostaining for LTBP4 and fibrillin-1.
Control and mutant fibroblasts (I:IV-6, C:II-2 and E:II-1) were stained for LTBP4 (green) and fibrillin-1 (FBN1; red). Nuclei were counterstained in blue. Fibroblasts from patient I:IV-6 show abnormal morphology of fibrillin-1 microfibrils and altered localization of LTBP4 to fibrillin-1. Fibroblasts from patients C:II-2 and E:II-1 lack LTBP4 staining in the extracellular matrix. Magnification bars: 50 µm.
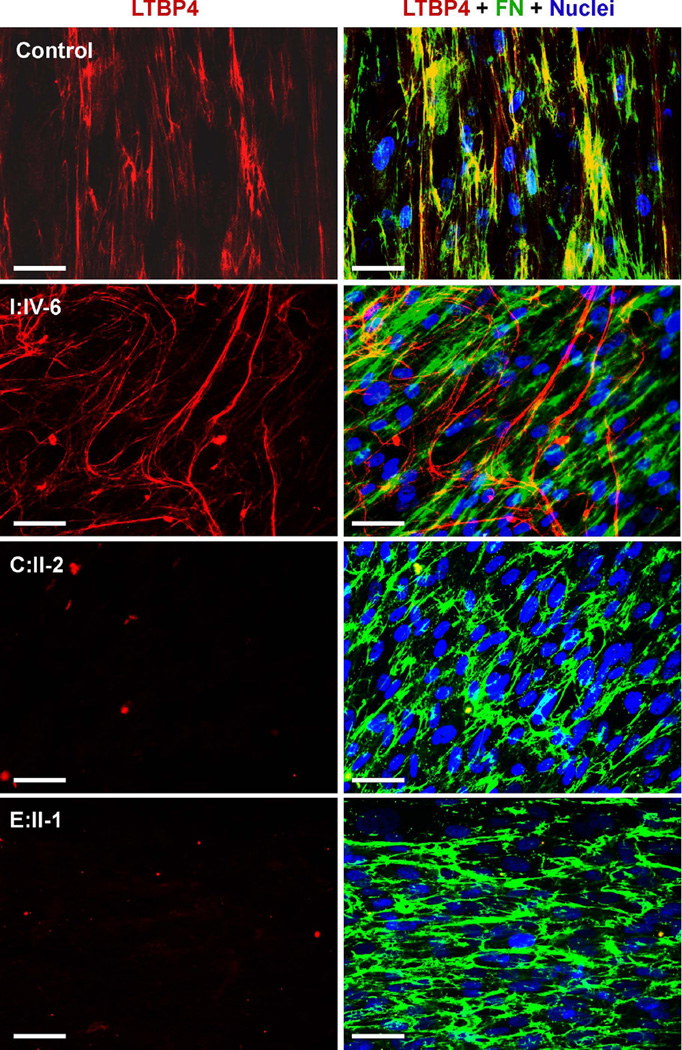
We reasoned that complete colocalization of the truncated LTBP4 with fibrillin-1 may be caused by the inability of this mutant molecule to bind another extracellular matrix component. Indeed, dual staining showed significantly reduced colocalization of the mutant LTBP4 with fibronectin (Figure 4) in most extracellular fibril networks, whereas wild-type LTBP4 showed patchy colocalization with fibronectin. These findings suggest that the C-terminus contributes to the recruitment of LTBP4 to fibronectin fibrils in cell-based systems.
Figure 4. The distribution of fibronectin and LTBP4 in fibroblast extracellular matrix.
Green color, red color and yellow color indicate fibronectin (FN), LTBP4 and colocalization, respectively. Nuclei were counterstained in blue. Control cells show patchy colocalization of LTBP4 with fibronectin. Cells from I:IV-6 deposit largely distinct fibronectin and LTBP4-containing networks. Cells from C:II-2 and E:II-I show no matrix staining for LTBP4. Magnification bars: 50µm.
Double staining for LTBP1 and fibrillin-1 was normal in patients C.II-2 and E.II-1 (Figure 5). In contrast, fibroblasts from patient I:IV-6 showed the same abnormally thick and wavy pattern of fibrillin-1 staining as previously. However, instead of complete colocalization observed between LTBP4 and fibrillin-1 in this patient, the colocalization between LTBP1 and fibrillin-1 had the same patchy pattern as in control cells.
Figure 5. Immunofluorescence staining for LTBP1 and fibrillin-1.
Control and patient (I:IV-6, C:II-2 and E:II-1) fibroblasts were stained for LTBP1 (red) and fibrillin-1 (FBN1; green). Nuclei were counterstained in blue. The same patchy colocalization pattern is observed between LTBP1 and fibrillin-1 in both control and mutant cells. However, microfibril bundles are thicker and wavier in fibroblasts form I:IV-6. Magnification bars: 100 µm.
Because significant phenotypic similarities exist between patients with FBLN5 and LTBP4 mutations we tested if LTBP4 mutations result in mislocalization of fibulin-5. Fibroblasts from patient E.II-1 showed normal localization of fibulin-5 to fibrillin-1 microfibrils (Supp. Figure S3), and fibulin-5 staining was normal in the other patients as well (data not shown).
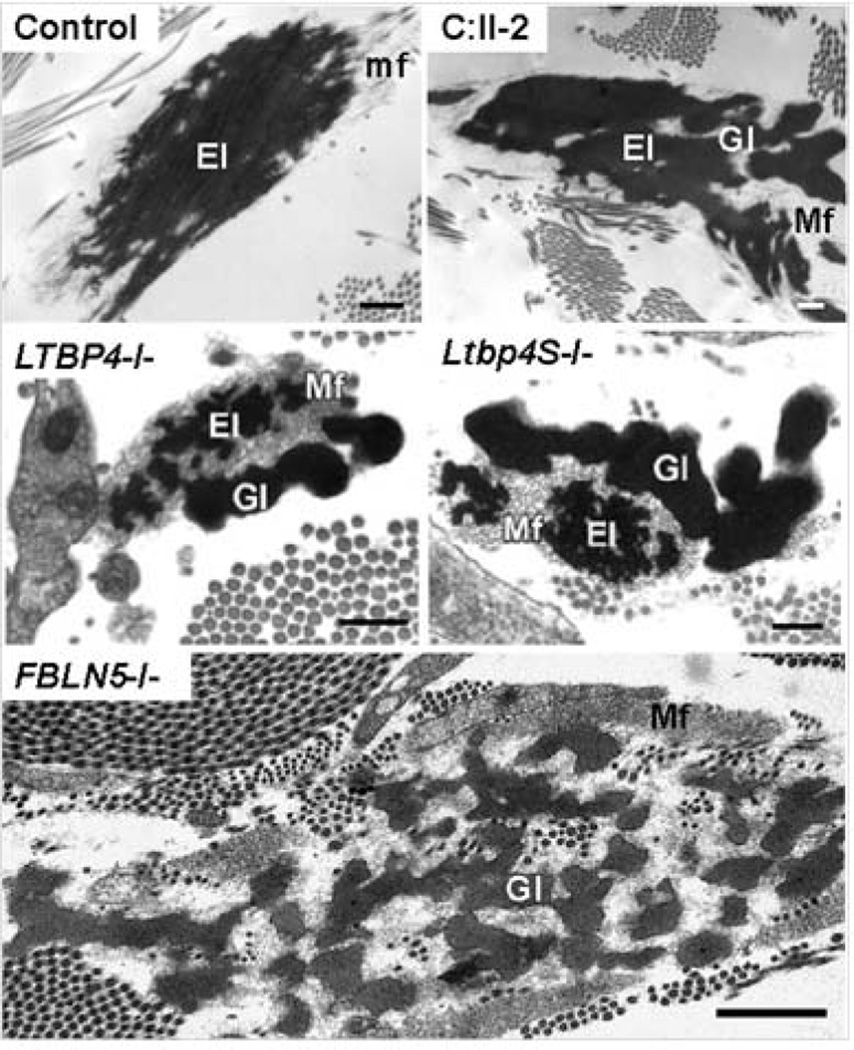
LTBP4 mutations cause peculiar electron microscopic anomalies of elastic fibers characterized by large, rounded deposits of elastin poorly integrated with microfibrils (Urban, et al., 2009). Electron microscopy of a skin biopsy specimen from participant C.II-2 showed the same type of elastic fiber abnormality as seen previously in patient and mice with homozygous LTBP4 mutations (Figure 6). In patients with FBLN5 mutations, elastic deposits are also rounded and poorly integrated with microfibrils, but the overall size of these abnormal elastin deposits are smaller and more uniform compared to patients with LTBP4 mutations (Figure 6).
Figure 6. Electron microscopic findings in LTBP4 and FBLN5 related cutis laxa.
In a control skin biopsy, electron microscopy of the elastic fiber consists of an elastin core (e) surrounded by microfibrils (mf), which form physical continuity and the same directionality as peripheral processes of the elastin core. In the patient (C:II-2), the elastin core (e) consists of globular deposits, which are poorly connected to each other and to peripheral microfibrils (mf). This abnormal elastic fiber morphology is similar to a previously published patient with a homozygous LTBP4 mutation (LTBP4−/−) and a knockout mouse for the short form of ltbp4 (Ltbp4S−/−). Images (LTBP4−/−) and (Ltbp4S−/−) were taken from (Urban, et al., 2009). Electron microscopic abnormalities of elastic fibers in a patient with a homozygous FBLN5 mutations (FBLN5−/−) showed small rounded elastin deposits poorly connected to microfibrils. Image (FBLN5−/−) was taken from (Hu, et al., 2006). Magnification bars: Control, C:II-2, LTBP4−/−: 500 nm; LTBP4S−/−: 200 nm; FBLN5−/− 1000 nm.
Increased TGFβ1 activity in LTBP4 mutant cells
We co-cultured reporter mink lung epithelial cells (MLECs) (Abe, et al., 1994) and fibroblasts from patients C:II-2, E:II-1 and I:IV-6 and four controls in order to measure the activity of TGFβ released by the fibroblasts. Patient cells released significantly higher levels of active TGFβ than did controls (p= 0.0001; patient vs. controls: 33.851ng/ml and 17.573 ng/ml, respectively). Because there was no difference in TGFβ1 expression among the 4 control fibroblasts (p= 0.621), we pooled the corresponding data representing it as “controls” in Supp. Figure S4. In contrast to patients C:II-2 and E:II-1, TGFβ1 level of Patient I:IV-6 fibroblasts cells was not statistically different from the control group (p=0.108). Given that greater reduction in LTBP4 expression at the mRNA level (Figure 2A) and the protein level (Figure 2D) was observed in patients C:II-2 and E:II-1 than in patient I:IV-6, we conclude that TGFβ1 activity in LTBP4 mutant cells inversely correlates with the residual amount of LTBP4 protein produced by the cells.
DISCUSSION
This study provides new molecular and clinical insights in a patient group with a phenotype reminiscent of ARCL type 1. FBLN5 mutations were rare (2/12, 17%), while LTBP4 were found in 9 out of 12 probands (75%). In the remaining proband that presented mainly with cutis laxa and bladder diverticula without obvious emphysema, no mutation in any of the genes known to be associated with ARCL type I was detected. Mutations might have been missed if situated in the promoter and regulating sequences that were not analyzed or due to technical imperfection. However, this patient may be affected by a related cutis laxa phenotype presenting with bladder diverticula without major pulmonary involvement that might be caused by mutations in genes that remain to be identified. As such, in patients with ARCL type 1 and emphysema, we advise to analyze LTBP4 in the absence of clear cardiovascular impairment, followed by FBLN5 if no mutation is identified. Alternatively, in case of cardiovascular involvement with arterial tortuosity and aneurysms, FBLN4 analysis seems most appropriate. We have summarized the clinical data for the subtypes of type I recessive cutis laxa in Supp. Table S3,in which we also included arterial tortuosity syndrome, caused by bi-allelic mutations in the SLC2A10 gene, because of considerable overlap with FBLN4 related cutis laxa.
Our data show that considerable clinical overlap exists between LTBP4 related cutis laxa, eponymously named Urban-Rifkin-Davis Syndrome, and FBLN5 deficient ARCL. Cutis laxa was mostly generalized and severe in both groups, but was more variable with milder features, later onset, or less facial involvement in some patients with LTBP4 mutations. In both entities, it is important to acknowledge the poor prognosis. Pulmonary manifestations are equally present, show variable severity and represent the most life threatening manifestation in both entities. This contrasts with FBLN4 deficient cutis laxa where vascular complications can severely reduce life expectancy and skin features are often less pronounced (Dasouki, et al., 2007; Renard, et al., 2010). Also, in the cardiovascular system, both LTBP4 deficient and FBLN5 deficient patients may present with peripheral pulmonary artery stenosis, a nonspecific feature that also occurs in related entities as FBLN4 deficiency and arterial tortuosity syndrome (Callewaert, et al., 2008; Renard, et al., 2010). Supravalvular aortic stenosis has been reported in FBLN5 deficiency (Elahi, et al., 2006; Loeys, et al., 2002), but not yet in LTBP4 related cutis laxa. Although bladder diverticula have been reported in some FBLN5 deficient patients (Loeys, et al., 2002; Van Maldergem, et al., 1988), diverticula are more severe and prevalent in LTBP4 deficient patients, where they are found both in the gastrointestinal and genitourinary tract and may progress to organ rupture. Rectal prolapse, gastrointestinal tortuosity and pelvic insufficiency also point to LTBP4 deficiency. Diaphragmatic hernia also shows higher prevalence in patients with LTBP4 mutations. Pyloric stenosis, a trait classically described with multifactorial inheritance (Krogh, et al., 2010), segregated with FBLN5 deficient cutis laxa in one family. However, the consanguinity in this family does not enable us to prove the causal relation of this trait with FBLN5 deficiency. Moreover, two previously reported patients with LTBP4 mutations had pyloric stenosis as well (Urban, et al., 2009), suggesting a more general role for correct elastic fiber assembly in the pathogenesis. Finally, no apparent central nervous system involvement, mental retardation or skeletal abnormalities, findings specific for ARCL type II, were found.
We identified one novel and one previously described mutation in the FBLN5 gene. The recurrent p.Cys217Arg mutation was also found in a Lebanese family making a common ancestor likely (Claus, et al., 2008). To the best of our knowledge, this report brings the number of FBLN5 mutations reported to date to 4 in a total of 6 families of which at least two had a common ancestor (Claus, et al., 2008; Elahi, et al., 2006; Loeys, et al., 2002; Nascimento, et al., 2010) confirming that this entity is extremely rare.
The majority of the LTBP4 mutations identified in this study resulted in premature termination of the LTBP4 open reading frame. qPCR analysis confirmed reduced mRNA levels, probably through NMD. Lower LTBP4 levels in the extracellular matrix may impair correct sequestration and controlled release of TGFβ as previously suggested (Urban, et al., 2009). Moreover, missense mutations identified in LTBP4 lead to the loss of one of the highly conserved cysteine residues located in a TB or hybrid domain, implicated in binding of the SLC. Loss or generation of these cysteine residues were shown to interfere with the conformation and function both in LTBP and fibrillin proteins (Jensen, et al., 2009; Lack, et al., 2003). This mechanism would connect to comparable disturbance of the TGFβ signaling pathway in the pathogenesis of related cutis laxa phenotypes including arterial tortuosity syndrome, FBLN4 related and autosomal dominant cutis laxa (Callewaert, et al., 2011; Callewaert, et al., 2008; Renard, et al., 2010) and other connective tissue disorders including the Marfan (Neptune, et al., 2003) and Loeys-Dietz syndromes (Loeys, et al., 2005). Therefore, dysregulation of TGFβ signaling is a common downstream molecular pathway involved in many connective tissue disorders, underlining the close relationship between microfibrillar and elastic fiber integrity and TGFβ signaling. Phenotypic similarities between these connective tissue diseases may be related to altered TGFβ signaling, whereas differences may be caused by differences in distinct primary functions of these genes, such as mechanical support (Callewaert, et al., 2011; Hu, et al., 2010) or cellular metabolism (Willaert, et al., 2012).
Our functional studies uncovered important inter-individual mechanistic differences among patients with LTBP4 mutations and provide new insights into microfibril assembly. Two of 3 mutations studied resulted, as expected, in severely reduced LTBP4 mRNA and protein levels and significantly elevated TGFβ signaling. In contrast, mutation p.Arg1377Alafs*27 (Family I) partially escaped NMD, producing significant amounts of mutant LTBP4. This C-terminally truncated protein altered the structure of microfibril bundles by producing thicker and wavier structures. In addition, the colocalization patterns of the mutant LTBP4 with both fibrillin and fibronectin were abnormal. In normal fibroblasts LTBP4 showed patchy colocalization with both fibronectin and with fibrillin-1. In contrast, truncated LTBP4 showed more uniform colocalization with fibrillin-1 microfibrils and reduced colocalization with fibronectin. These findings suggest that the C-terminal region of LTBP4 is required for binding fibronectin but is not necessary for binding fibrillin-1 microfibrils.
Because loss-of-function mutations do not cause alterations in fibrillin-1 microfibril morphology, we propose that the C-terminal truncation mutation produced these alterations in a gain of function manner. Although the exact molecular mechanism remains unclear, we speculate that uniform binding of truncated LTBP4 to fibrillin-1 microfibrils may enhance lateral growth of microfibril bundles or impede their normal turnover leading to the observed thickening of the bundles. Because the C-terminus of LTBP4 is known to be required for cell attachment (Kantola, et al., 2008) the interaction of these abnormal bundles with cells may also be impaired. Interestingly, in family I, which carried the p.Arg1377Alafs*27 mutation, all patients had severe gastro-intestinal involvement. Thus, fibrillin-1 bundle-size or the ability of microfibrils to support cell attachment via LTBP4 may be particularly important for gastro-intestinal development.
In conclusion, we broadened the mutational spectrum in ARCL with emphysema in both the LTBP4 and FBLN5 genes and our results underscore extensive clinical overlap between Urban-Rifkin-Davis syndrome and ARCL type I. Our findings show that, in addition to loss-of-function, gain of function should also be considered as disease mechanisms initiated by LTBP4 mutations. Most mutations result in a LTBP4 loss of function mechanism and elevated TGFβ1 signaling. Other mutations with partial mutant protein expression implicate alterations of microfibrillar structures in the pathogenesis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Robert P. Mecham and Dr. Lynn Y. Sakai for providing antibodies.
GRANT SPONSORS
This work was supported in part by a Methusalem grant to ADP (BOF 08/01M01108 from the Ghent University), in part by the National Institutes of Health (RO1 HL090648 to ZU, P50 HL 084948 to FCS and UL1 RR024153 to the University of Pittsburgh Clinical and Translational Science Institute) and by the Division of Nephrology, Department of Internal Medicine, National Taiwan University Hospital, Yun-Lin branch, Taiwan to CTS. BC and FM are postdoctoral fellows of the Fund for Scientific Research – Flanders. OV has a BOF research fellowship from the Ghent University.
Footnotes
The authors have no conflict of interest to declare.
REFERENCES
- Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem. 1994;216(2):276–284. doi: 10.1006/abio.1994.1042. [DOI] [PubMed] [Google Scholar]
- Annes JP, Chen Y, Munger JS, Rifkin DB. Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. J Cell Biol. 2004;165(5):723–374. doi: 10.1083/jcb.200312172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell LS, Pitt J, Aftimos S, Ramadas R, Maw MA, Robertson SP. A missense mutation in ALDH18A1, encoding Delta1-pyrroline-5-carboxylate synthase (P5CS), causes an autosomal recessive neurocutaneous syndrome. Eur J Hum Genet. 2008;16(10):1176–1186. doi: 10.1038/ejhg.2008.91. [DOI] [PubMed] [Google Scholar]
- Callewaert B, Renard M, Hucthagowder V, Albrecht B, Hausser I, Blair E, Dias C, Albino A, Wachi H, Sato F others. New insights into the pathogenesis of autosomal-dominant cutis laxa with report of five ELN mutations. Hum Mutat. 2011;32(4):445–455. doi: 10.1002/humu.21462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callewaert BL, Willaert A, Kerstjens-Frederikse WS, De Backer J, Devriendt K, Albrecht B, Ramos-Arroyo MA, Doco-Fenzy M, Hennekam RC, Pyeritz RE others. Arterial tortuosity syndrome: clinical and molecular findings in 12 newly identified families. Hum Mutat. 2008;29(1):150–158. doi: 10.1002/humu.20623. [DOI] [PubMed] [Google Scholar]
- Claus S, Fischer J, Megarbane H, Megarbane A, Jobard F, Debret R, Peyrol S, Saker S, Devillers M, Sommer P others. A p.C217R mutation in fibulin-5 from cutis laxa patients is associated with incomplete extracellular matrix formation in a skin equivalent model. J Invest Dermatol. 2008;128(6):1442–1450. doi: 10.1038/sj.jid.5701211. [DOI] [PubMed] [Google Scholar]
- Dasouki M, Markova D, Garola R, Sasaki T, Charbonneau NL, Sakai LY, Chu ML. Compound heterozygous mutations in fibulin-4 causing neonatal lethal pulmonary artery occlusion, aortic aneurysm, arachnodactyly, and mild cutis laxa. Am J Med Genet A. 2007;143A(22):2635–2641. doi: 10.1002/ajmg.a.31980. [DOI] [PubMed] [Google Scholar]
- Davis EC. Stability of elastin in the developing mouse aorta: a quantitative radioautographic study. Histochemistry. 1993;100(1):17–26. doi: 10.1007/BF00268874. [DOI] [PubMed] [Google Scholar]
- de Cavanagh EM, Ferder M, Inserra F, Ferder L. Angiotensin II, mitochondria, cytoskeletal, and extracellular matrix connections: an integrating viewpoint. Am J Physiol Heart Circ Physiol. 2009;296(3):H550–H558. doi: 10.1152/ajpheart.01176.2008. [DOI] [PubMed] [Google Scholar]
- de Schepper S, Loeys B, de Paepe A, Lambert J, Naeyaert JM. Cutis laxa of the autosomal recessive type in a consanguineous family. Eur J Dermatol. 2003;13(6):529–5233. [PubMed] [Google Scholar]
- Elahi E, Kalhor R, Banihosseini SS, Torabi N, Pour-Jafari H, Houshmand M, Amini SS, Ramezani A, Loeys B. Homozygous missense mutation in fibulin-5 in an Iranian autosomal recessive cutis laxa pedigree and associated haplotype. J Invest Dermatol. 2006;126(7):1506–1509. doi: 10.1038/sj.jid.5700247. [DOI] [PubMed] [Google Scholar]
- Guernsey DL, Jiang H, Evans SC, Ferguson M, Matsuoka M, Nightingale M, Rideout AL, Provost S, Bedard K, Orr A others. Mutation in pyrroline-5-carboxylate reductase 1 gene in families with cutis laxa type 2. Am J Hum Genet. 2009;85(1):120–129. doi: 10.1016/j.ajhg.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Loeys BL, Coucke PJ, De Paepe A, Mecham RP, Choi J, Davis EC, Urban Z. Fibulin-5 mutations: mechanisms of impaired elastic fiber formation in recessive cutis laxa. Hum Mol Genet. 2006;15(23):3379–3386. doi: 10.1093/hmg/ddl414. [DOI] [PubMed] [Google Scholar]
- Hu Q, Shifren A, Sens C, Choi J, Szabo Z, Starcher BC, Knutsen RH, Shipley JM, Davis EC, Mecham RP others. Mechanisms of emphysema in autosomal dominant cutis laxa. Matrix Biol. 2010;29(7):621–628. doi: 10.1016/j.matbio.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucthagowder V, Sausgruber N, Kim KH, Angle B, Marmorstein LY, Urban Z. Fibulin-4: a novel gene for an autosomal recessive cutis laxa syndrome. Am J Hum Genet. 2006;78(6):1075–1080. doi: 10.1086/504304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen SA, Iqbal S, Lowe ED, Redfield C, Handford PA. Structure and interdomain interactions of a hybrid domain: a disulphide-rich module of the fibrillin/LTBP superfamily of matrix proteins. Structure. 2009;17(5):759–768. doi: 10.1016/j.str.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling MF, Mott JD, Finnegan MT, Jurukovski V, Erickson AC, Walian PJ, Taylor SE, Ledbetter S, Lawrence CM, Rifkin DB others. Isoform-specific activation of latent transforming growth factor beta (LTGF-beta) by reactive oxygen species. Radiat Res. 2006;166(6):839–848. doi: 10.1667/RR0695.1. [DOI] [PubMed] [Google Scholar]
- Kaler SG, Gallo LK, Proud VK, Percy AK, Mark Y, Segal NA, Goldstein DS, Holmes CS, Gahl WA. Occipital horn syndrome and a mild Menkes phenotype associated with splice site mutations at the MNK locus. Nature genetics. 1994;8(2):195–202. doi: 10.1038/ng1094-195. [DOI] [PubMed] [Google Scholar]
- Kantola AK, Keski-Oja J, Koli K. Fibronectin and heparin binding domains of latent TGF-beta binding protein (LTBP)-4 mediate matrix targeting and cell adhesion. Exp Cell Res. 2008;314(13):2488–2500. doi: 10.1016/j.yexcr.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Koli K, Myllarniemi M, Keski-Oja J, Kinnula VL. Transforming growth factor-beta activation in the lung: focus on fibrosis and reactive oxygen species. Antioxid Redox Signal. 2008;10(2):333–342. doi: 10.1089/ars.2007.1914. [DOI] [PubMed] [Google Scholar]
- Kornak U, Reynders E, Dimopoulou A, van Reeuwijk J, Fischer B, Rajab A, Budde B, Nurnberg P, Foulquier F, Lefeber D others. Impaired glycosylation and cutis laxa caused by mutations in the vesicular H+-ATPase subunit ATP6V0A2. Nat Genet. 2008;40(1):32–34. doi: 10.1038/ng.2007.45. [DOI] [PubMed] [Google Scholar]
- Krogh C, Fischer TK, Skotte L, Biggar RJ, Oyen N, Skytthe A, Goertz S, Christensen K, Wohlfahrt J, Melbye M. Familial aggregation and heritability of pyloric stenosis. JAMA. 2010;303(23):2393–2399. doi: 10.1001/jama.2010.784. [DOI] [PubMed] [Google Scholar]
- Lack J, O’Leary JM, Knott V, Yuan X, Rifkin DB, Handford PA, Downing AK. Solution structure of the third TB domain from LTBP1 provides insight into assembly of the large latent complex that sequesters latent TGF-beta. J Mol Biol. 2003;334(2):281–291. doi: 10.1016/j.jmb.2003.09.053. [DOI] [PubMed] [Google Scholar]
- Leao-Teles E, Quelhas D, Vilarinho L, Jaeken J. De Barsy syndrome and ATP6V0A2-CDG. Eur J Hum Genet. 2009 doi: 10.1038/ejhg.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KG, Bercovitch L, Dill SW, Robinson-Bostom L. Acquired disorders of elastic tissue: Part II. decreased elastic tissue. J Am Acad Dermatol. 2004;51(2):165–185. doi: 10.1016/j.jaad.2004.03.016. quiz 186–8. [DOI] [PubMed] [Google Scholar]
- Loeys B, Van Maldergem L, Mortier G, Coucke P, Gerniers S, Naeyaert JM, De Paepe A. Homozygosity for a missense mutation in fibulin-5 (FBLN5) results in a severe form of cutis laxa. Hum Mol Genet. 2002;11(18):2113–2118. doi: 10.1093/hmg/11.18.2113. [DOI] [PubMed] [Google Scholar]
- Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, Meyers J, Leitch CC, Katsanis N, Sharifi N others. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet. 2005;37(3):275–281. doi: 10.1038/ng1511. [DOI] [PubMed] [Google Scholar]
- Markova D, Zou Y, Ringpfeil F, Sasaki T, Kostka G, Timpl R, Uitto J, Chu ML. Genetic heterogeneity of cutis laxa: a heterozygous tandem duplication within the fibulin-5 (FBLN5) gene. American journal of human genetics. 2003;72(4):998–1004. doi: 10.1086/373940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S, Washko GR, Yamashiro T, Estepar RS, Diaz A, Silverman EK, Hoffman E, Fessler HE, Criner GJ, Marchetti N others. Pulmonary hypertension and computed tomography measurement of small pulmonary vessels in severe emphysema. Am J Respir Crit Care Med. 2010;181(3):218–225. doi: 10.1164/rccm.200908-1189OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento GM, Nunes CS, Menegotto PF, Raskin S, Almeida N. Cutis laxa: case report. An Bras Dermatol. 2010;85(5):684–686. doi: 10.1590/s0365-05962010000500013. [DOI] [PubMed] [Google Scholar]
- Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33(3):407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- Renard M, Holm T, Callewaert B, Adès L, Baspinar O, Pickart A, Dasouki M, Rauch A, Coucke P, Dietz H, De Paepe A, Loeys B. Major cardiovascular involvement in autosomal recessive cutis laxa patients confirms fibulin-4 as a key player in vascular elastic fiber formation. Belgium: Center for Medical Genetics, Ghent university Hospital; 2010. [Google Scholar]
- Renard M, Holm T, Veith R, Callewaert BL, Ades LC, Baspinar O, Pickart A, Dasouki M, Hoyer J, Rauch A others. Altered TGFbeta signaling and cardiovascular manifestations in patients with autosomal recessive cutis laxa type I caused by fibulin-4 deficiency. Eur J Hum Genet. 2010;18(8):895–901. doi: 10.1038/ejhg.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reversade B, Escande-Beillard N, Dimopoulou A, Fischer B, Chng SC, Li Y, Shboul M, Tham PY, Kayserili H, Al-Gazali L others. Mutations in PYCR1 cause cutis laxa with progeroid features. Nat Genet. 2009 doi: 10.1038/ng.413. [DOI] [PubMed] [Google Scholar]
- Szabo Z, Crepeau MW, Mitchell AL, Stephan MJ, Puntel RA, Yin Loke K, Kirk RC, Urban Z. Aortic aneurysmal disease and cutis laxa caused by defects in the elastin gene. Journal of medical genetics. 2006;43(3):255–258. doi: 10.1136/jmg.2005.034157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassabehji M, Metcalfe K, Hurst J, Ashcroft GS, Kielty C, Wilmot C, Donnai D, Read AP, Jones CJ. An elastin gene mutation producing abnormal tropoelastin and abnormal elastic fibres in a patient with autosomal dominant cutis laxa. Human molecular genetics. 1998;7(6):1021–1028. doi: 10.1093/hmg/7.6.1021. [DOI] [PubMed] [Google Scholar]
- Urban Z, Gao J, Pope FM, Davis EC. Autosomal dominant cutis laxa with severe lung disease: synthesis and matrix deposition of mutant tropoelastin. J Invest Dermatol. 2005;124(6):1193–1199. doi: 10.1111/j.0022-202X.2005.23758.x. [DOI] [PubMed] [Google Scholar]
- Urban Z, Hucthagowder V, Schurmann N, Todorovic V, Zilberberg L, Choi J, Sens C, Brown CW, Clark RD, Holland KE others. Mutations in LTBP4 cause a syndrome of impaired pulmonary, gastrointestinal, genitourinary, musculoskeletal, and dermal development. Am J Hum Genet. 2009;85(5):593–605. doi: 10.1016/j.ajhg.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Maldergem L, Vamos E, Liebaers I, Petit P, Vandevelde G, Simonis-Blumenfrucht A, Bouffioux R, Kulakowski S, Hanquinet S, Van Durme P others. Severe congenital cutis laxa with pulmonary emphysema: a family with three affected sibs. Am J Med Genet. 1988;31(2):455–464. doi: 10.1002/ajmg.1320310226. [DOI] [PubMed] [Google Scholar]
- Willaert A, Khatri S, Callewaert BL, Coucke PJ, Crosby SD, Lee JG, Davis EC, Shiva S, Tsang M, De Paepe A others. GLUT10 is required for the development of the cardiovascular system and the notochord and connects mitochondrial function to TGFbeta signaling. Human molecular genetics. 2012;21(6):1248–1259. doi: 10.1093/hmg/ddr555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor beta1 - an intimate relationship. Eur J Cell Biol. 2008;87(8–9):601–615. doi: 10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179(6):1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.