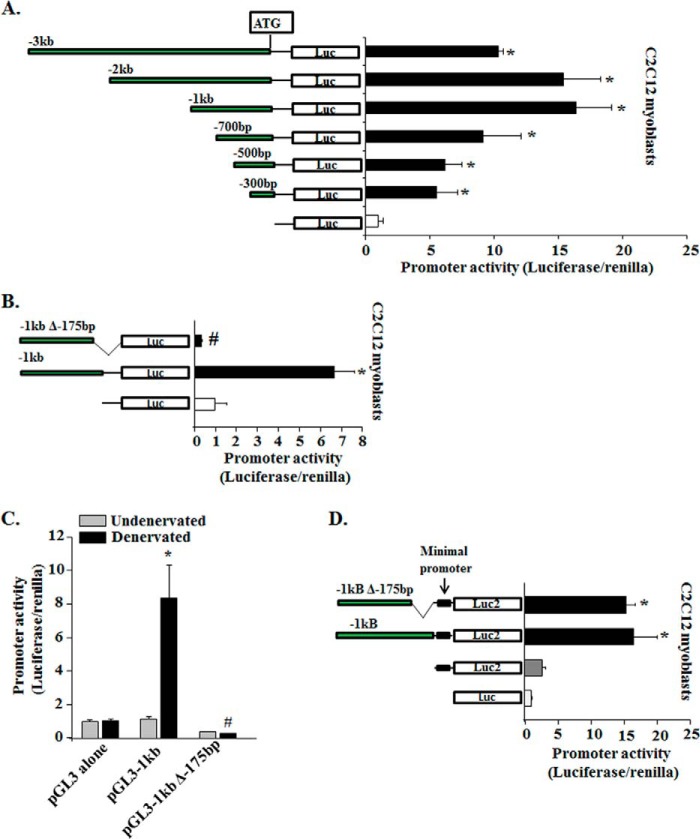
FIGURE 2.
Characterization of mouse Fn14 promoter in vitro and in vivo. A, a series of 5′-deleted fragments (−3 to −0.3 kb) of the murine Fn14 promoter was generated and cloned at the upstream of the luciferase coding sequence into the promoter-less pGL3 plasmid and transfected into C2C12 myoblasts along with Renilla plasmid (in 1:50 ratio). Schematic representation of the promoter fragments analyzed (left panel). The promoter-driven luciferase activities were measured after 48 h of myoblast transfection. The results were normalized with an empty vector (n = 4). *, p < 0.01; values vary significantly from cells transfected with empty vector (pGL3 alone). B, C2C12 myoblasts were transfected with pGL3–1kb or pGL3–1kbΔ-175bp plasmids along with Renilla plasmid, and luciferase activity was measured 48 h later (n = 4). *, p < 0.01; values vary significantly from C2C12 myoblasts transfected with empty vector. #, p < 0.01; values vary significantly from C2C12 myoblasts transfected with pGL3–1kb plasmid. C, fold change in luciferase activity (normalized using Renilla luciferase) in TA muscle transfected with empty vector (pGL3), pGL3–1kb, or pGL3–1kbΔ-175bp plasmid after 3 days of denervation (n = 3 in each group). *, p < 0.01; values vary significantly from TA muscle transfected with empty vector. #, p < 0.01; values vary significantly from TA muscle transfected with pGL3–1kb plasmid. D, C2C12 myoblasts were transfected with empty pGL3, empty pGL4.23, pGL4.23–1kb, or pGL4.23–1kbΔ-175bp plasmids along with Renilla plasmid and luciferase activity was measured 48 h later (n = 4). *, p < 0.01; values vary significantly from C2C12 myoblasts transfected with empty vectors. Error bars represent S.D.