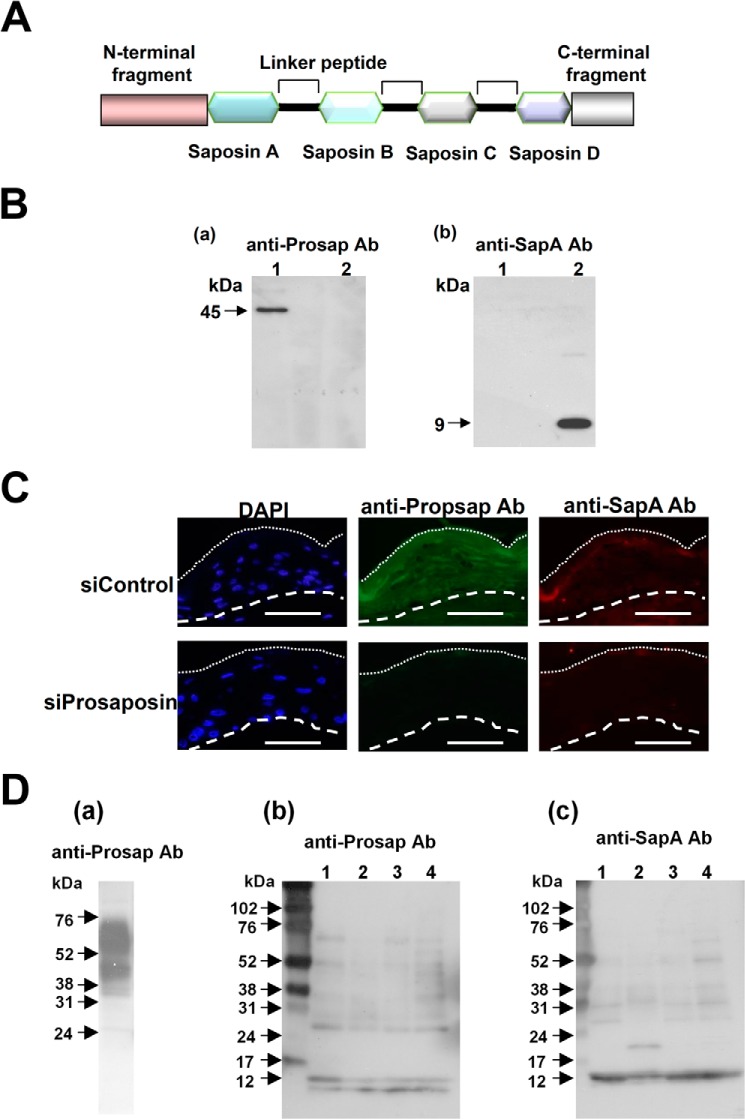
FIGURE 1.
Detection of prosaposin and saposin A. A, a schematic illustration of prosaposin structure is shown. B, reactivity of anti-Prosap and anti-SapA antibodies (Ab). Specificities of these antibodies were tested with recombinant prosaposin (lane 1) and saposin A (lane 2). Western blot analysis was carried out using antibodies to prosaposin (anti-Prosap Ab) and saposin A (anti-SapA Ab). Anti-Prosap Ab recognized a 45-kDa band that is a non-glycosylated form of prosaposin. Anti-SapA Ab recognized saposin A at 9 kDa. C, immunohistochemical analysis of the antibody specificity. Skin equivalent models were constructed after treatment with nonspecific siRNA control (siControl) and specific siRNA to prosaposin (siProsaposin). Sections were stained with anti-Prosap Ab or anti-SapA Ab. Nuclear staining with DAPI was also shown. Dotted line shows the border of the cornified layer. Broken line indicates the epidermal-dermal junction. Scale bars: 50 μm. D, presence of prosaposin and saposin A in cornified cell extracts. a, extracts from a skin equivalent model were used as a prosaposin control. Heavy staining of the 65- (glycosylated form) and 45-kDa bands were evident with some degradation products. b, extracts from cornified cells of healthy subjects (lanes 1–4) were subjected to Western blotting using anti-Prosap Ab. Anti-Prosap Ab recognized the liberated N-terminal fragments with 10 and 12 kDa as well as intermediate degradation products. c, the presence of saposin A in the same extracts was tested with anti-SapA Ab. Each lane contained 10 μg of extracts.