FIGURE 1.
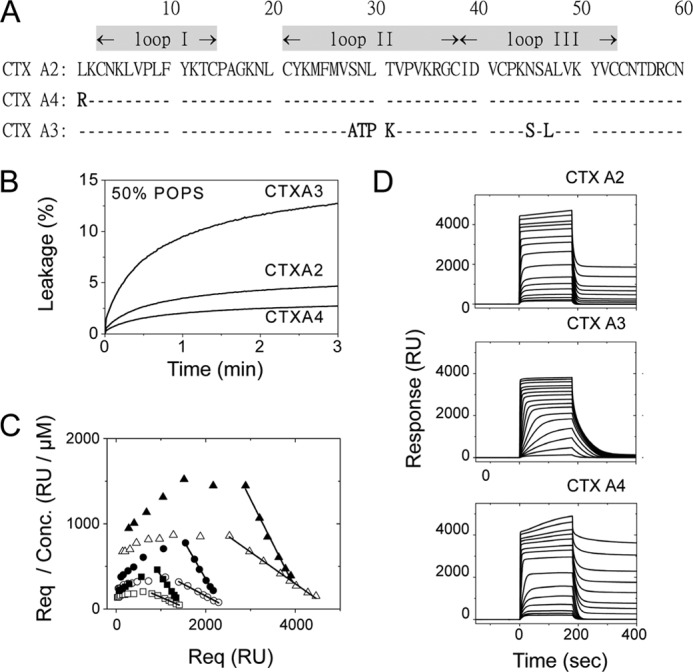
Heparin and negatively charged lipid as binding targets for three CTX homologues with a well defined spatial distribution of the positively charged domain. A, primary sequences of CTX homologues show high homologies but vary in several positively charged residues at the N-terminal and loop II regions. B, vesicle leakage assay on different CTX homologues shows differences in specificity toward negatively charged lipids. Time-dependent leakage of 6-CF dyes from 50% POPS vesicles was induced by CTX (0.16 μm). The higher affinity of CTX A3 toward the negatively charged lipids, POPS, was indicated by a higher leakage content, but this was not seen for CTX A2 or A4 under the same conditions. C, Scatchard plots (Req/concentration (Conc.) versus Req) for CTX A2 (open symbols) or CTX A4 (closed symbols) in an SPR binding assay (0.15–8 μm) on immobilized heparins under different surface densities (triangle, 700 RU; circle, 350 RU; square, 200 RU) were shown. D, SPR binding of CTX homologues (0.15–20 μm) onto immobilized heparin surfaces (700 RU as representative plots) showed different retention capabilities. Significant CTX retention on immobilized heparin surfaces was observed for CTX A2 and A4 at higher concentrations but not for CTX A3.
