Background: Reelin controls many aspects of brain development and function.
Results: Purified Reelin activates Erk1/2 signaling and gene expression, but previously identified receptors and adaptor molecules are not required for these activities.
Conclusion: Activation of Erk1/2 signaling by Reelin occurs through a novel signaling mechanism.
Significance: Reelin induces Erk/1/2 signaling and thus promotes events that are required for neuronal maturation.
Keywords: Cell Culture, Cell Signaling, Extracellular Signal-regulated Kinase (ERK), Neurodevelopment, Signal Transduction
Abstract
Reelin is an extracellular protein that controls many aspects of pre- and postnatal brain development and function. The molecular mechanisms that mediate postnatal activities of Reelin are not well understood. Here, we first set out to express and purify the full length Reelin protein and a biologically active central fragment. Second, we investigated in detail the signal transduction mechanisms elicited by these purified Reelin proteins in cortical neurons. Unexpectedly, we discovered that the full-length Reelin moiety, but not the central fragment, is capable of activating Erk1/2 signaling, leading to increased p90RSK phosphorylation and the induction of immediate-early gene expression. Remarkably, Erk1/2 activation is not mediated by the canonical signal transduction pathway, involving ApoER2/VLDLR and Dab1, that mediates other functions of Reelin in early brain development. The activation of Erk1/2 signaling likely contributes to the modulation of neuronal maturation and synaptic plasticity by Reelin in the postnatal and adult brain.
Introduction
Reelin is an extracellular protein that plays multiple roles in brain development and function (1). In the prenatal forebrain Reelin governs radial neuronal migration and cellular layer formation (2–4). In the postnatal forebrain Reelin stimulates dendrite outgrowth, branching of entorhinohippocampal terminals, synapse formation, and synaptic plasticity (5–12). Excitatory neurons of the forebrain are the best-characterized cellular targets of Reelin, which critically depend on the presence of this factor for radial migration and synaptic maturation (13, 14). These cells express key components of the Reelin signal transduction machinery, including the two high-affinity receptors, apolipoprotein E receptor 2 (ApoER2)3 and very low-density lipoprotein receptor (VLDLR), and the essential adaptor protein Disabled-1 (Dab1) (15–18). Reelin binding to ApoER2/VLDLR receptors activates Src family kinases (SFKs), which phosphorylate Dab1 at specific tyrosine residues (19–22). The phosphorylated Dab1 further activates multiple downstream signaling pathways, including Crk/Rap1 signaling affecting cell adhesion (13, 23–26), and phosphatidylinositol-3 kinase (PI3K)/Akt and mTOR signaling, which promotes dendrite outgrowth and spine formation (9, 27–30). Finally, a splicing variant of ApoER2, Dab1, and the NMDA receptor have been shown to participate in the control of synaptic activity, plasticity and cognitive function by Reelin (5, 30, 31). However, the signaling mechanisms that underlie these functions are not completely understood.
Reelin is a large, modular glycoprotein containing 8 unique repeats. Some of the secreted full-length Reelin is cleaved by extracellular proteases into three major fragments: an N-terminal fragment, a central fragment, and a C-terminal fragment (29). The central fragment alone can bind ApoER2 and VLDLR, induce Dab1 phosphorylation and activate Dab1-dependent downstream signaling events leading to layer formation in cortical slice cultures (32, 33). However, the full-length protein has been shown to be more potent than the central fragment alone, likely due to the presence of the N-terminal region, which promotes multimerization (34, 35), and the C-terminal region, which also contributes to the full activity (36). Finally, uncleaved Reelin has been shown to be more potent than the cleaved protein as a result of reduced clearance and prolonged Dab1 signaling (37).
Since the initial cloning of the Reelin gene, enormous progress has been made to elucidate the functions of this protein in brain development. However, a detailed molecular analysis of Reelin signal transduction has been hindered by the difficulty of obtaining workable amounts of purified protein. Thus, most studies so far relied on the use of conditioned medium containing unpurified, heterogeneous Reelin proteins. In this study, we first overcame this technical limitation by generating large amounts of purified full-length Reelin and its central fragment. Second, we re-examined signal transduction in cultured cortical neurons. Our study reveals a novel activity of Reelin that is specifically induced by the full-length moiety and leads to the activation of Erk1/2 signaling and immediate-early gene expression through a non-canonical signaling pathway that does not involve lipoprotein receptors.
EXPERIMENTAL PROCEDURES
Animal Handling
Animals used in this study were handled in accordance with a protocol approved by the Association for Assessment and Accreditation of Laboratory Animal Care AAALAC committee at Rutgers, the State University of New Jersey. Wild type mice (ICR mice, Taconic Farms) were used for the isolation of cortical neurons. Mutant mouse strains were Reeler mice (B6C3Fe-ala-Relnrl/+) (The Jackson Laboratories) and Dab1 KO mice (a gift of J. A. Cooper, Fred Hutchinson Cancer Research Center).
Reelin Expression and Purification
Full-length mouse Reelin cDNA (FL) was cloned into a modified pCMV6-XL4 vector (38). Reelin proteins tagged at the C terminus with a human Fc region were expressed in HEK293 GnTI- cells and stable lines were selected using G418 (Geneticin, Sigma) as described previously (38). Secreted Reelin proteins were purified from the culture medium using a protein A column (Captiv-A PriMab affinity resin by RepliGen). The Fc tag was removed after chromatography by overnight cleavage with 3C Protease at 4 °C. Affinity-purified proteins were buffer exchanged and concentrated up to ∼4–6 mg/ml with Microsep centrifugal devices (Pall Corporation). Mass spectrometry analysis of purified Reelin was performed as described in supplemental Experimental Procedures.
Primary Neuronal Culture and Treatment
Cerebral cortices were dissected from the brain of embryonic day (E) 15.5–18.5 ICR mice, and neurons were dissociated using a Papain Dissociation Kit (BioWorthington). Neurons were cultured in 6-well plates coated with poly-l-lysine in Neurobasal medium supplemented with 2% B-27 supplement, 0.5 mm l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). Glutathione S-transferase (GST) and glutathione S-transferase receptor-associated protein (GST-RAP) were isolated as described previously (9). Cortical neurons were treated with purified Reelin in the presence or absence of LY294002 (Cell Signaling), U0126 (Cell Signaling), PP2 (Calbiochem), GST, or GST-RAP. All inhibitors were added 15–30 min prior to Reelin treatment.
Immunoprecipitation and Western Blot Analysis
Primary cortical neurons or freshly dissected brain tissue were lysed in RIPA buffer. For immunoprecipitation assay, the lysates were incubated with anti-Dab1 rabbit antibodies (Rockland) at 4 °C overnight, and precipitated with protein G-agarose beads (Pierce). For Western blotting, samples were loaded onto 8% or 10% SDS-PAGE gels and transferred to 0.22-μm nitrocellulose membranes. The membranes were incubated with blocking buffer, followed by primary antibodies overnight at 4 °C, and secondary antibodies for 1 h at room temperature. Membranes were developed with ECL-Plus Western blotting Detection System (GE Healthcare). Primary antibodies were: mouse monoclonal anti-Dab1 (L2; a gift from Dr. André M Goffinet, Université Catholique de Louvain, Belgium), mouse anti-phospho-tyrosine 4G10 (Millipore), rabbit anti-phospho-Akt Ser-473 (Cell Signaling), rabbit anti-total Akt (Cell Signaling), rabbit anti-phospho-Erk1/2 (Cell Signaling), rabbit anti-total Erk1/2 (Cell Signaling), rabbit anti-Arc (Santa Cruz Biotechnology), and mouse anti-β-actin-HRP (Sigma). Secondary antibodies were HRP-conjugated (Sigma).
Immunofluorescence
Dissociated cortical neurons grown on glass coverslips coated with poly-l-lysine were fixed in 4% paraformaldehyde (PFA), permeabilized with 0.2% Triton X-100, and blocked with 5% normal goat serum for 1 h at room temperature. Cells were incubated with rabbit anti-phospho-p90RSK Thr-573 antibody (Cell Signaling) and mouse anti-Map2 (Covance) at 4 °C overnight, followed by secondary antibodies conjugated to AlexaFluor 488 or AlexaFluor 647 (Invitrogen) for 1 h at room temperature. Cells were imaged by confocal microscopy using a Yokogawa CSU-10 spinning disk. To measure the percentage of double-labeled neurons, 14–15 confocal images containing 20–30 neurons per image were analyzed.
mRNA Isolation and Quantitative RT-PCR Analysis
mRNA was isolated from cultured neurons using RNeasy mini kit (Qiagen) and used to generate cDNA using high-capacity cDNA reverse transcription kit (Applied Biosystems). Real-time quantitative PCR was performed with transcript-specific primers as described in supplemental Experimental Procedures. The Pfaffl method was used for calculation of relative quantification, and the gene expression was normalized against the expression level of gapdh (39).
Statistical Analysis
Data in the plots are shown as the mean ± S.E., and analyzed by Student's t test or one-sample t test as indicated in the figure legends. The results were averaged from multiple experiments. Statistical significance was determined when p < 0.05.
RESULTS
Expression and Purification of Full-length Reelin and Its Central Fragment
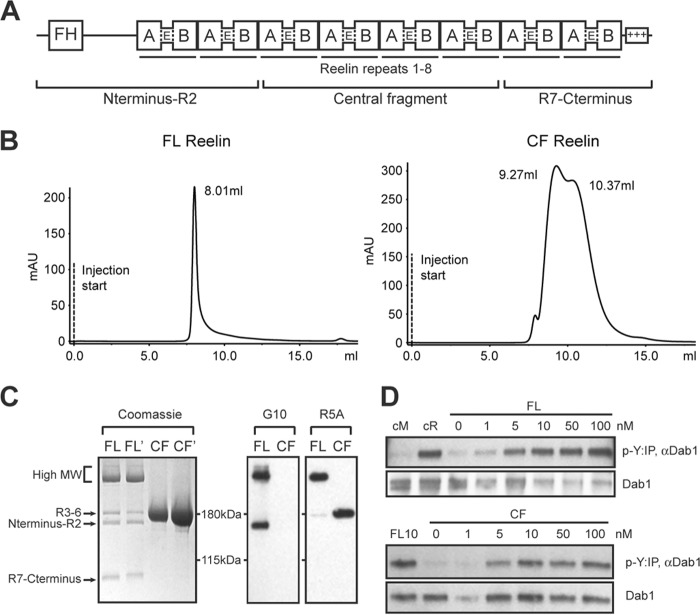
To produce full-length (FL) Reelin and its central fragment (CF) R3–6 (Fig. 1A), HEK293 GnTI- cells were transfected with expression constructs and Reelin proteins were purified from the conditioned medium by affinity chromatography. HEK293 GnTI- cells maintain a minimal glycosylation that homogenously adds a Man5GlcNAc2 group (molecular mass, 1234 Da) that is sufficient to properly fold many recombinant proteins (38). Since the molecular weight (MW) of the FL Reelin protein is 387 kDa, containing 20 potential N-linked glycosylation sites, the expected size of the glycosylated product is predicted to be ∼411 kDa. The yield of expression for both Reelin constructs was ∼2 mg of purified protein/liter culture medium. Freshly purified Reelin proteins were analyzed by size exclusion chromatography using Superdex 200 10/300GL. FL Reelin was eluted as a single peak with a mass corresponding to >650 kDa (Fig. 1B). Because the resin utilized does not have the resolution to separate particles larger than this molecular weight, the elution profile suggests that freshly purified FL Reelin is mostly intact, and is likely to form dimers or oligomers as previously reported (33). CF Reelin was eluted as a mixture of two species, with MWs that are consistent with the formation of dimers (∼320 kDa) and tetramers (∼620 kDa) (Fig. 1B). Purified Reelin proteins were further analyzed to assess their stability, cleavage, and biochemical activity. Coomassie staining of proteins separated by SDS-PAGE revealed that FL Reelin undergoes some degree of proteolytic cleavage within few days after purification, whereas the CF remained stable (Fig. 1C). The FL Reelin moiety contained a predominant broad band of high MW (∼350–400 kDa) corresponding to predicted full-length isoform and intermediate cleavage fragments, and three fainter bands corresponding to fully cleaved N-terminal (N-R2, 180 kDa), the central (R3–6, 190 kDa), and C-terminal (R7-C, 80 kDa) fragments. These fragments are likely generated by two proteolytic cleavage events, occurring between Reelin repeats (R) R2-R3, and R6-R7 (Fig. 1A), as previously described for unpurified Reelin conditioned medium (29, 37, 40). These cleavage events could be the result of spontaneous protein processing or they could be due to the activity of miniscule amounts of proteases that co-purify with FL Reelin. Western blot analysis with Reelin monoclonal antibodies G10, directed against an N-terminal epitope, or R5A, directed against the central fragment, further demonstrated that the purified FL Reelin moiety contained mostly high MW isoforms and fully cleaved fragments of the expected size (Fig. 1C). The purified CF isoform appeared as a single band of ∼190 kDa, consistent with the size of the central fragment generated from the cleavage of unpurified Reelin protein (29), and was readily detectable with the R5A antibody (Fig. 1C).
FIGURE 1.
Purification and analysis of full-length Reelin and its central fragment. A, domain structure of Reelin. Reelin contains an F-spondin homology (FH) region at N terminus, 8 repeats, and a positively charged C terminus. Each repeat contains subdomains A and B, separated by an epidermal growth factor (E)-like motif. Full-length Reelin is cleaved into three fragments. B, Full-length Reelin (FL) and central fragment (CF) proteins secreted in the culture medium of HEK293 GnTI- cells were separated by size exclusion chromatography using Superdex 200 10/300GL. FL Reelin eluted as a single peak with a MW >650 kDa, whereas CF Reelin exhibited two peaks compatible with the formation of a dimer (∼320 kDa) and a tetramer (∼620 kDa). C, Coomassie staining and Western blot analysis of purified proteins separated by SDS-PAGE. Two different batches of FL and CF Reelin were analyzed by Coomassie staining. FL Reelin contained a major band of high MW (∼350–400 kDa), and cleaved fragments of the expected size. Purified CF appeared as a single band of ∼190 kDa, corresponding to the central fragment. Western blot analysis of purified proteins with the N-terminal antibody G10 detects high MW and the N-terminal fragment of FL Reelin. The R5A antibody directed against the central fragment detects high MW and the central fragment of FL Reelin, as well as CF Reelin. D, primary cortical neurons were treated with purified FL and CF Reelin at the indicated concentrations and subjected to the Dab1 phosphorylation assay. Reelin conditioned medium (cR) and mock medium (cM) were used as controls. Both Fl and CF proteins induced robust Dab1 phosphorylation (p-Y).
To further characterize the molecular composition of the high MW FL Reelin band we performed in-gel trypsin digestion and orthogonal liquid chromatography tandem mass spectrometry peptide analysis (LCLC-MS/MS). We identified 215 Reelin peptides from more 1100 mapped MS/MS spectral counts that were well distributed throughout the peptide sequence. Mapping the identified peptides to the known Reelin amino acid sequence indicated a high degree of sequence coverage throughout the protein starting from F34 through R3398, confirming the presence of full-length Reelin protein (supplemental Fig. S1). Previous studies demonstrated that both, FL Reelin and the CF induce the phosphorylation of Dab1 on tyrosine residues in cultured cortical neurons (32, 33). This biochemical event is essential for many aspects of Reelin activity in brain development. To determine whether our purified Reelin proteins retain biological activity, we treated primary cortical neurons with increasing concentrations of the FL or the CF protein, and performed the Dab1 tyrosine phosphorylation assay (Fig. 1D). The results show that both FL and CF proteins induce robust Dab1 phosphorylation. The activity of purified FL Reelin at the 100 nm concentration was similar to that of unpurified conditioned medium obtained from a previously described 293T stable cell line (CER) (9). Purified CF was also active in this assay, however its potency appeared to be somewhat reduced compared with FL, consistent with previous reports (33).
Differential Regulation of Akt and Erk Signaling Pathways by Purified FL and CF Reelin Proteins
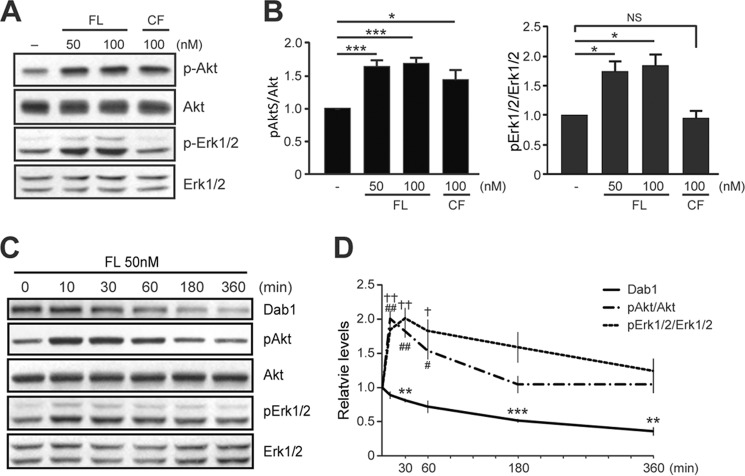
Previous studies revealed that exposure of cultured neurons to Reelin conditioned medium induces Akt phosphorylation in a Dab1-dependent manner (27, 28, 41). Other studies implicated Reelin in Erk1/2 activation in adult subventricular neuronal cultures (42), but not in developing forebrain neurons (27). To re-examine the effects of Reelin on Akt and Erk1/2 signaling using purified proteins, we exposed mouse cortical neurons to FL or CF Reelin proteins. Western blot analysis revealed that both Reelin proteins consistently activated Akt signaling, as indicated by the increased phosphorylation of Akt at Ser-473 (Fig. 2, A and B) and Thr-308 (data not shown). FL Reelin resulted in ∼1.6-fold increase in Akt phosphorylation compared with control buffer, whereas CF Reelin caused an ∼1.4-fold increase. Surprisingly, purified FL Reelin also significantly induced Erk1/2 phosphorylation at the same concentrations, whereas CF Reelin had no effect (Fig. 2, A and B). The increase in Erk1/2 phosphorylation by FL Reelin was slightly higher than that of Akt phosphorylation at the 20 min time point examined. To gain a better understanding of the signaling mechanisms elicited by FL Reelin, we examined the time course of Akt and Erk1/2 phosphorylation. Both, Akt and Erk1/2 phosphorylation peaked between 10 and 30 min; however the levels of phospho-Erk1/2 appeared to be more sustained than those of Akt (Fig. 2, C and D). While Akt phosphorylation returned to approximately basal levels within 3 h, Erk1/2 phosphorylation remained significantly elevated at this time point and returned to approximately basal levels by 6 h. Given that many cellular events are dependent on sustained Erk activation, including growth and differentiation by neurotrophins (43, 44), these results suggest that FL Reelin-induced Erk1/2 activation may significantly affect neuronal maturation.
FIGURE 2.
Activation of Akt and Erk signal transduction pathways by FL Reelin in dissociated cortical neurons. A, 5 DIV mouse cortical neurons were exposed to purified Reelin for 20 min and assayed by Western blotting. Both FL and CF Reelin induced Akt phosphorylation (Ser-473), but only FL Reelin induced Erk1/2 phosphorylation (Thr-202/Tyr-204). B, data were quantified from five independent experiments. The graphs show mean ± S.E. C and D, Time course of Akt and Erk1/2 phosphorylation by FL Reelin and quantification of the results from multiple experiments.
Akt and Erk1/2 Signaling Abnormalities in Juvenile Heterozygous Reeler Mice and Dab1 Knock-out Mice
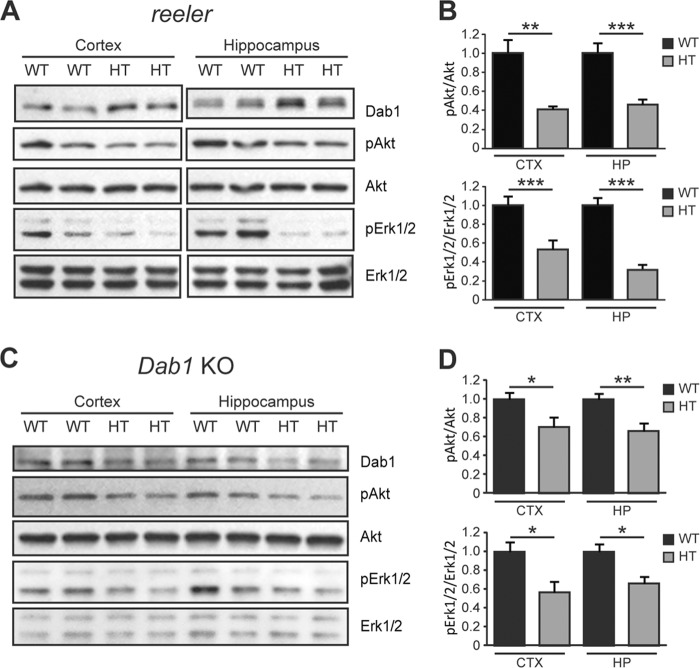
To determine whether Reelin and Dab1 signaling affect the activity of Akt and Erk1/2 pathways in vivo we analyzed the brain of reeler and constitutive Dab1 knock out (KO) mice. Loss of Reelin in homozygous reeler mice leads to severe developmental brain malformations, whereas Reelin deficiency in heterozygous reeler mice lead to synaptic and behavioral abnormalities without causing gross anatomical defects (10, 45–47). Similarly, homozygous constitutive Dab1 KO mice exhibit a reeler-like behavioral and anatomical phenotype, whereas heterozygous mice appear normal but have subtle dendrite and synaptic abnormalities (10, 17). Thus, heterozygous mice models are appropriate to investigate mechanisms underlying specifically postnatal brain development and function. To determine whether reduced Reelin levels affect the activity of PI3K- and MEK-dependent pathways in vivo, we analyzed forebrain lysates from WT and heterozygous reeler mice at pre-and postnatal ages. Western blot analysis revealed that Akt phosphorylation is unaffected at prenatal ages (data not shown), but is significantly reduced in the cerebral cortex as well as the hippocampus of heterozygous reeler mice at postnatal, juvenile ages (3–4 weeks; n = 7–9 mice per genotype) (Fig. 3, A and B). Similarly, Erk1/2 phosphorylation was unaffected at prenatal ages (data not shown), but was significantly reduced in the cerebral cortex as well as the hippocampus of heterozygous reeler mice at juvenile ages (Fig. 3, A and B). These striking results strongly suggest that Reelin plays an important role in the modulation of both Akt and Erk1/2 signaling in the postnatal brain.
FIGURE 3.
Abnormal Akt and Erk1/2 signaling in juvenile heterozygous reeler and Dab1 KO mice. A, Western blot analysis of forebrain regions from 3–4-week-old wild type (WT) and heterozygous (HT) reeler mice. The levels of phospho-Akt and phospho-Erk1/2 were significantly reduced both the cerebral cortex and hippocampus of reeler mice. B, data were quantified from n = 9 WT and n = 7 HT mice of the reeler strain. C, Western blot analysis of cortex and hippocampus from 3–4-week-old WT and HT Dab1 KO mice. The phosphorylation levels of Akt and Erk1/2 were decreased significantly in HT mice. D, data were quantified from n = 10 WT, n = 7 HT mice of the Dab1 KO strain.
To examine whether Dab1 deficiency also causes signaling abnormalities in vivo, we analyzed the forebrain of WT and heterozygous Dab1 KO mice. We found that the basal phosphorylation levels of Akt and Erk1/2 in both the cerebral cortex and the hippocampus were significantly reduced in juvenile heterozygous Dab1 KO mice compared with WT (n = 7–10 mice per genotype) (Fig. 3, C and D). The extent of Akt and Erk1/2 basal signaling reduction was slightly smaller in heterozygous Dab1 KO mice than in heterozygous reeler mice, suggesting that Dab1 may be partially involved in modulating the effects of Reelin on these signaling pathways. Together, the observation that Akt and Erk1/2 signaling pathways are dramatically suppressed in heterozygous reeler as well as heterozygous Dab1 KO mice, in the absence of an overt neuroanatomical phenotype, and that they are specifically disrupted at late developmental postnatal ages, suggest that these molecular abnormalities may be related to postnatal activities in synaptic function and plasticity.
Molecular Mechanisms of Akt and Erk Activation by FL Reelin
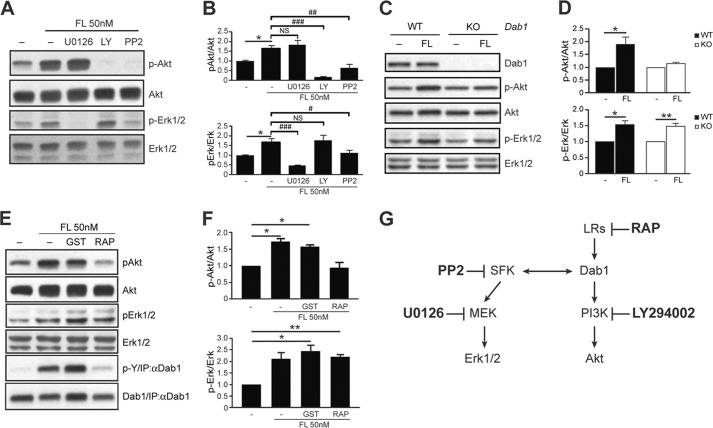
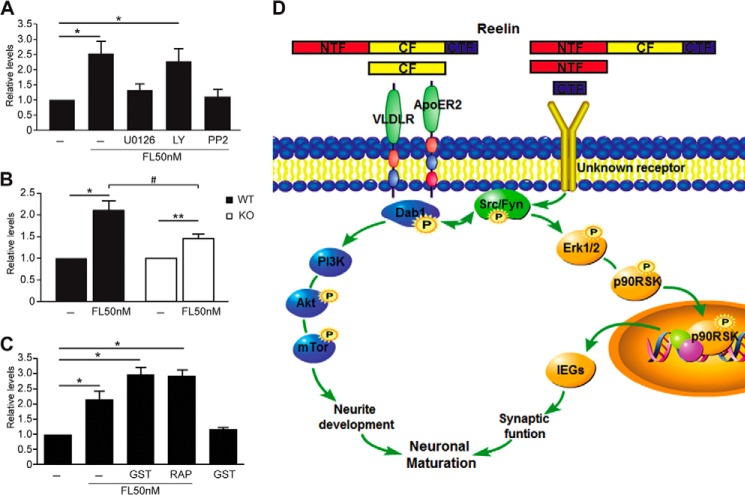
To investigate the molecular mechanisms mediating FL Reelin-induced Akt and Erk1/2 signaling, we first exposed cortical neurons to FL Reelin in the presence or absence of a MEK inhibitor (U0126), a PI3K inhibitor (LY294002), or a Src family kinase (SFK) inhibitor (PP2) (Fig. 4G). Western blot analysis demonstrated that Reelin-induced Akt phosphorylation is not affected by U0126, but is completely abolished by LY294002 or PP2 treatment (Fig. 4, A and B), confirming that this event is PI3K- and SFK-dependent, but independent of MEK signaling. Reelin-induced Erk1/2 phosphorylation, on the other hand, was completely abolished by U0126 and was significantly reduced by PP2, but was unaffected by LY294002 treatment, demonstrating that Erk1/2 activation is dependent on SFK and MEK, but is independent of PI3K signaling. Together, these results indicate that SFKs play a central role in Reelin signal transduction, and that two distinct branches, a PI3K- and a MEK-dependent signaling cascade, convey Reelin signaling to downstream effectors in cortical neurons.
FIGURE 4.
Activation of Erk1/2 signaling by FL Reelin is independent of the ApoER2/VLDLR-Dab1 canonical pathway. A, Western blot analysis of Reelin-treated 5 DIV cortical neurons in the presence or absence of pharmacological inhibitors. The induction of phospho-Akt by Reelin was not affected by the MEK inhibitor U0126, but was abolished by the PI3K inhibitor LY294002 (30 μm) and by the SFK inhibitor PP2 (10 μm). The induction of phospho-Erk1/2 by Reelin was abolished by U0126 (10 μm) and PP2, but was not affected by LY294002. B, data were quantified from 4–5 independent experiments. C, WT and Dab1 KO cortical neurons were cultured for 5 DIV. FL Reelin induced Akt phosphorylation in WT cortical neurons, but not in Dab1-deficient neurons. Reelin-induced Erk1/2 phosphorylation in both, WT and Dab1 KO neurons. D, data were quantified from n = 3 WT, n = 5 KO independent cultures. E, binding to ApoER2/VLDLR receptors is not required for Reelin-induced Erk1/2 activation. Cortical neurons were treated with FL Reelin in the presence or absence of GST-bound lipoprotein receptor antagonist (RAP) or GST alone as control. Both proteins were added at the 50 μg/ml concentration 15 min prior to Reelin exposure. Akt activation by FL Reelin was completely blocked by RAP, whereas Erk1/2 activation was not affected. F, data were quantified from three independent experiments. G, diagram of the targets of the pharmacological inhibitors used in these experiments. All graphs show mean ± S.E.
SFKs are well known to phosphorylate the adaptor protein Dab1 in response to stimulation with Reelin-conditioned medium (19, 20, 22). Phospho-Dab1 in turn promotes further SFK activation and mediates PI3K and Akt activation (20). To investigate the role of Dab1 in the activation of PI3K- and MEK-dependent pathways by purified FL Reelin, we cultured cortical neurons from homozygous Dab1 KO embryos and their WT littermates, and exposed them to FL Reelin. As expected, FL Reelin induced Akt phosphorylation in WT, but not in Dab1-deficient cortical neurons (Fig. 4, C and D). In contrast, FL Reelin induced Erk1/2 phosphorylation in both, WT and Dab1 KO neurons (Fig. 4, C and D). These results demonstrate that Dab1 is absolutely required for PI3K-Akt activation, but not for MEK-Erk1/2 activation by FL Reelin. To investigate the potential involvement of upstream components of the canonical Reelin signaling pathway in Erk1/2 activation, we treated cortical neurons with FL Reelin in the presence of the lipoprotein receptor antagonist receptor-associated protein (RAP) fused to GST. This reagent effectively blocks Reelin binding to ApoER2 and VLDLR, thereby inhibiting many of its biological and biochemical activities (9, 48, 49). GST alone was used as a control. When cortical neurons were treated with FL Reelin in the presence of GST-RAP, Akt phosphorylation was completely suppressed, whereas Erk1/2 phosphorylation was not affected (Fig. 4, E and F). To confirm the effectiveness of GST-RAP inhibition, we also examined Dab1 tyrosine phosphorylation, and found that it was completely blocked by this treatment, as expected (Fig. 4E). Together, our data demonstrate that FL Reelin induces Erk1/2 phosphorylation through a novel mechanism that is independent of the canonical ApoER2/VLDLR-Dab1 signaling pathway but requires SFK and MEK activity (Fig. 7D).
FIGURE 7.
IEGs induction by FL Reelin is mediated by SFKs and MEK. A, quantitative RT-PCR of Reelin-treated cortical neurons in the presence or absence of pharmacological inhibitors. Arc mRNA induction by FL Reelin was completely blocked by U0126 (10 μm) and PP2 (10 μm), but not by LY294002 (30 μm). B, Dab1 is partially required for Reelin-induced Arc mRNA expression. Cortical neurons isolated from newborn Dab1 KO or WT mice. The induction of Arc mRNA by FL Reelin was reduced in KO compared with WT neurons. C, ApoER2/VLDLR are not required for FL Reelin-induced IEG expression. Cortical neurons were incubated with GST-RAP to block Reelin binding to ApoER2/VLDLR receptors, or to GST alone as control. Arc mRNA induction by Reelin was not affected by RAP treatment. D, schematic diagram illustrating signal transduction pathways activated by FL Reelin.
FL Reelin Induces p90RSK Phosphorylation through the Erk1/2 Pathway
To identify downstream events that follow activation of Erk1/2 signaling by FL Reelin, we examined the phosphorylation of p90 ribosomal S6 kinase (p90RSK) at Thr-573. The phosphorylation if this residue is carried out by phospho-Erk1/2 and represents the earliest step in a sequential phosphorylation cascade that results in p90RSK activation (50, 51). Activated phospho-p90RSK then translocates to the nucleus where it regulates the transcription of many Erk1/2-dependent genes. Cortical neurons were treated with FL Reelin or control buffer for 20 min, and processed for immunofluorescence using phospho-p90RSK antibodies. Cultures were counterstained with Map2 antibodies to specifically identify neuronal cells. In both control and Reelin-treated cultures the phospho-p90RSK signal was detected in the nucleus of a subset of Map2-positive neurons, as expected. However, Reelin treatment significantly increased the number of phospho-p90RSK-positive neurons compared with control (Fig. 5, A and B). Indeed, the percentage of phospho-p90RSK-positive neurons almost doubled after Reelin treatment compared with control. To examine the mechanism of Reelin-induced p90RSK phosphorylation, cortical neurons were incubated with the PI3K inhibitor LY294002 or the MEK inhibitor U0126 for 30 min before treatment. Treatment with LY294002 did not affect Reelin induction of p90RSK phosphorylation, whereas treatment with U0126 almost completely abolished p90RSK phosphorylation under control or Reelin-stimulated conditions (Fig. 5B). These results demonstrate that FL Reelin induces the phosphorylation of p90RSK at Thr-573 through a mechanism that involves MEK-Erk1/2 activity and is independent of PI3K signaling.
FIGURE 5.
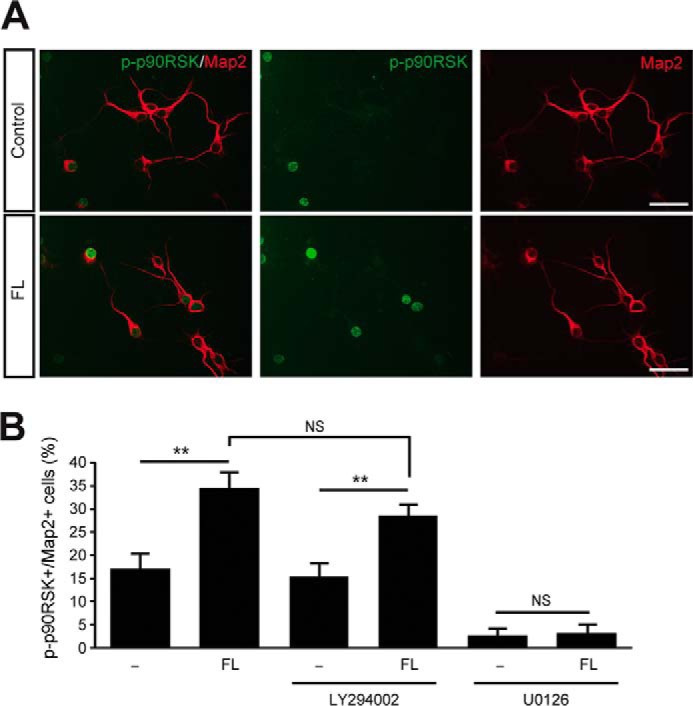
Induction of p90RSK phosphorylation by FL Reelin in cortical neurons. A, representative confocal images of cortical neurons treated with control buffer or FL Reelin and processed for immunofluorescence using antibodies against phospho-p90RSK (green) and Map2 (red). B, Reelin significantly increased the percentage of double labeled neurons in the absence of inhibitors and in the presence of LY294002. U0126 pretreatment virtually abolished basal and Reelin-induced levels of phospho-p90RSK. n = 315–345 neurons from 14–15 visual fields per treatment. Scale bars, 50 μm. **, p < 0.01.
FL Reelin Induces the Expression of Activity-dependent Immediate Early Genes
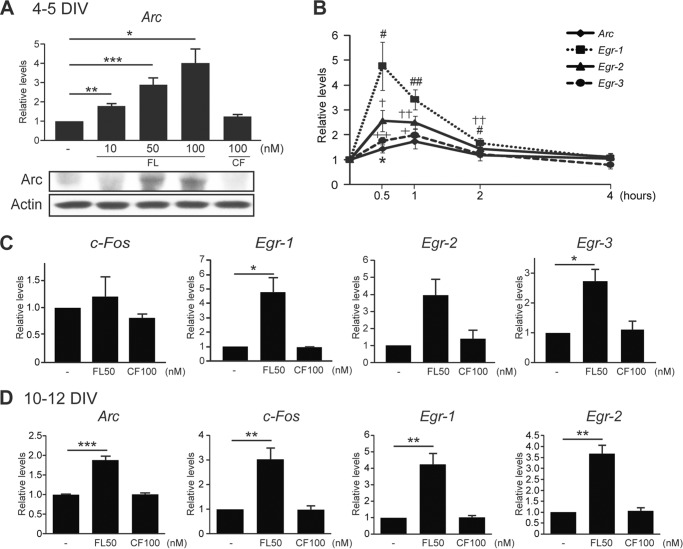
Neuronal activity and neurotrophins are known to induce the Erk1/2-dependent expression of several immediate early genes (IEGs). Some of these genes, such as the activity-regulated cytoskeleton-associated (Arc) encode proteins that are involved in the modulation of cytoskeletal dynamics and synaptic plasticity (52, 53). p90RSK crucially mediates the expression of many Erk1/2-dependent IEGs, including c-Fos and members of the early-response gene family (Egr), and directly phosphorylates some IEG products (51, 54, 55). To determine whether Reelin affects Arc expression in developing cortical neurons, we first treated immature primary cultures at 4–5 DIV with FL or CF Reelin for 1 h and examined Arc mRNA levels by quantitative RT-PCR. We found that FL Reelin induced Arc expression in a concentration-dependent manner, whereas CF had no effect (Fig. 6A). The maximal expression of Arc mRNA was induced by 100 nm FL Reelin treatment. To determine whether the observed Arc mRNA induction resulted in increased protein levels we also analyzed protein extracts from parallel cultures exposed to similar concentrations of FL or CF Reelin for 3 h. Western blot analysis reveals that Arc protein levels are increased after treatment with 50–100 nm FL Reelin, but not with CF Reelin (Fig. 6A). Similar results were obtained in two independent replicate experiments (not shown). The induction of Arc transcripts by FL Reelin was detectable at 30 min, peaked around 1 h, and returned to normal level within 4 h after treatment, in a time course that is very similar to that reported in response to neuronal activity stimulation (56) (Fig. 6C). To determine whether Reelin affects the expression of other activity-dependent IEGs, we next examined the expression of c-Fos and members of the Egr family of transcription factors. In these immature cultures, we found that 1 h treatment with FL Reelin did not significantly affect c-Fos expression, but it significantly induced the expression of all Egr family members examined (Fig. 6, B and C). In more mature cultures (10–12 DIV), 1 h treatment with FL Reelin significantly induced the expression of all IEGs examined, including c-Fos (Fig. 6D). As for Arc, CF Reelin did not affect the expression of IEGs at any time point (Fig. 6, B and D, and data not shown). A similar induction of IEGs by FL Reelin was also seen in fully mature cortical neurons (18–19 DIV), but not in very young neurons (2–3 DIV) (data not shown). Together, the data suggest that FL Reelin is increasingly capable of stimulating activity-dependent IEG expression during the course of neuronal maturation in vitro.
FIGURE 6.
Induction of IEGs by FL Reelin. Cortical neurons were treated with FL or CF Reelin for 1 h and mRNA was extracted for quantitative RT-PCR. A, in 4–5 DIV cultures FL Reelin induced Arc mRNA expression in a concentration-dependent manner (10–100 nm), whereas CF Reelin did not. The immunoblot shown below demonstrates induction of Arc protein expression after 3 h of stimulation with higher concentrations of FL, but not CF Reelin. B, FL Reelin induces Egr family of transcription factors, but not c-Fos in 4–5 DIV cortical neurons. CF Reelin has no effect. C, time course of Arc and Egr1–3 mRNA expression by FL Reelin 50 nm. D, in 10–12 DIV neurons, FL Reelin induced the expression of all IEGs examined. *, p < 0.05; **, p < 0.01. ***, p < 0.001.
FL Reelin Induces IEG Expression via a SFKs-Erk1/2-dependent Pathway
Next, we investigated the signal transduction mechanisms that mediate Arc mRNA induction by FL Reelin. Cortical neurons were treated with FL Reelin as described above with or without pharmacological inhibitors, and analyzed by quantitative RT-PCR. The data show that Arc induction by Reelin was blocked by the MEK inhibitor U0126, and by the SFK inhibitor PP2, but not by the PI3K inhibitor LY294002 (Fig. 7A). To test the role of Dab1, we used cortical cultures derived from Dab1-deficient KO and WT mice. These data revealed that the absence of Dab1 results in an attenuated, but not completely abolished, response to FL Reelin (Fig. 7B), suggesting that Dab1 partially affects IEG induction. Finally, we investigated the involvement of ApoER2/VLDLR receptors using the GST-RAP competitive inhibitor. The data show that induced Arc mRNA levels were not affected by the presence of this inhibitor (Fig. 7C), suggesting that lipoprotein receptors are not involved in the up-regulation of this gene.
Together, the data strongly suggest that FL Reelin induces IEG expression through a signaling pathway that includes SFK, MEK, and Erk1/2, but not ApoER2/VLDLR receptors or PI3K/Akt signaling (Fig. 7D). Dab1 contributes to this event, but it is not absolutely required.
DISCUSSION
Numerous studies demonstrated that the canonical ApoER2/VLDLR/Dab1 signaling pathway mediates many of the functions of Reelin in pre- and postnatal brain development and function. However, in this study we discovered that an additional, non-canonical pathway mediates at least some postnatal functions of Reelin. In particular, we documented the SFK- and MEK-dependent induction of Erk1/2, p90RSK, and IEGs by FL Reelin. This series of events appears to be particularly evident at late stages of neuronal maturation, when synaptogenesis and the modulation of synaptic activity predominantly occur. Furthermore we showed that, while CF Reelin is sufficient to activate the canonical pathway leading to Dab1 and Akt phosphorylation, this fragment alone does not activate the non-canonical Erk1/2 pathway. Thus, either an uncleaved full-length Reelin isoform, or a different fragment contained in the FL moiety, induces this activity through a yet unidentified receptor. Finally, we showed that both Akt and Erk1/2 signaling are defective in the postnatal brain of heterozygous reeler and Dab1 mutant mice. All the data gathered in the present study can be interpreted in a model in which Reelin elicits the activation of two signal transduction branches through distinct protein domains that engage different receptors. The CF binds lipoprotein receptors resulting in PI3K/Akt activation, whereas the N-terminal fragment, the C-terminal fragment or the uncleaved FL Reelin protein binds an unidentified receptor that results in MEK/Erk1/2 activation (Fig. 7D). Crosstalk between the two signaling cascades occurs at the level of SFKs, which are required for both branches of the pathway. Because Dab1 phosphorylation promotes further activation of SFKs in a positive feedback loop, this may explain why it is partially involved in MEK/Erk1/2 activation by Reelin.
The purification strategy employed here enabled us, for the first time, to examine in detail the time course and the concentration-dependent activation of specific signal transduction pathways by Reelin. It also enabled us to conduct pharmacological experiments to probe components of the signaling machinery, and their role in p90RSK phosphorylation and IEG induction. Thus, the availability of a purified reagent led us to the identification of a novel signaling cascade that is consistently induced by the FL Reelin moiety. The identity of the Reelin receptor that mediates Erk1/2 activation is presently unknown, but the failure of the lipoprotein receptor antagonist RAP to inhibit this event suggests that a different class of receptors may be involved. Previous studies suggested the existence of Reelin receptors or co-receptors other than ApoER2 and VLDLR. For example, early binding experiments implicated β1 integrins in Reelin signaling (57). However, the analysis of mutant mice suggested that β1 integrins are not essential for Reelin signaling in neurons (58), but rather function primarily in radial glia (59, 60). Other studies reported Reelin binding to Cadherin-related neuronal receptor (CNR) family, particularly CNR1 (61), a finding that has been disputed by other investigators (32). Other potential non-lipoprotein Reelin receptors include ephrin B proteins and the interacting EphB transmembrane tyrosine kinases, which were reported to bind the N-terminal region of Reelin (62, 63). Because CNR1, ephrin B and EphB are expressed in neurons and associate with the SFKs, they could conceivably mediate the induction of Erk1/2 signaling. However, preliminary experiments (not shown) do not support the involvement of any of these putative Reelin receptors. Clearly, additional biochemical, genetic, and functional studies will be required to conclusively identify the receptors that mediate Reelin induction of Erk1/2 and IEG expression in neurons.
The induction of Erk1/2 phosphorylation by purified FL Reelin reported in this study is consistent with a previous report, in which Reelin-conditioned medium was shown to activate these MAP kinases in postnatal subventricular zone cells (42). Other investigations, however, failed to detect Erk1/2 activation by Reelin conditioned medium in cortical neurons (27). Possible explanations for the discrepancy with our findings include the age of the cultures or the composition of the Reelin reagent. In our hands, we were able to detect modest Erk1/2 activation by Reelin conditioned medium compared with mock conditioned medium (not shown), although the conditioned medium itself increased basal levels of phosphorylation. Our data are also consistent with the previous report that Reelin induces some IEGs in a manner that is dependent on the serum response factor (SRF) (64). Indeed, together these findings strongly support the view that the activation of Erk1/2 and p90RSK by Reelin, through SRF and IEGs, promotes neuronal maturation. Further supporting this concept, we demonstrated that both Akt and Erk1/2 signaling are profoundly reduced in vivo when Reelin or Dab1 levels are reduced, but only at postnatal ages. However, it should be noted that the significance of Erk1/2 activation by Reelin likely extends beyond the period of late postnatal development. Indeed, we recently demonstrated that Akt and Erk1/2 signaling are deficient in adult-specific conditional Dab1 knock out mice (31). Given that Erk1/2 signaling crucially regulates synaptic plasticity, and learning and memory (65–68), and that mutant mice with reduced Reelin-Dab1 signaling exhibit significant impairment in these functions (5, 31, 47), the present findings identify a molecular mechanism linking Reelin to the control of synaptic plasticity and memory formation in the postnatal and adult brain.
Supplementary Material
Acknowledgments
We thank Valentina Dal Pozzo and Beth Crowell for providing technical assistance with this work, Andre' Goffinet for Dab1 antibodies, and Jonathan Cooper for Dab1 KO mice. We would also like to thank the Robert Wood Johnson Foundation (Grant 67038) for their support of the Child Health Institute of New Jersey.
This study was supported in part by National Institutes of Health Grant R01-MH092906 (to D. C.) and by Research Grant 10-409-SCH-E-O from the New Jersey Governor's Council for Medical Research and Treatment of Autism (to G. D.).

This article contains supplemental Experimental Procedures and Fig. S1.
- apoER
- apolipoprotein E receptor
- VLDLR
- very low-density lipoprotein receptor
- SFK
- Src family kinase
- IEG
- immediate early gene
- FL
- full length
- CF
- central fragment
- CNR
- cadherin-related neuronal receptor
- SRF
- serum response factor.
REFERENCES
- 1. D'Arcangelo G. (2014) Reelin in the Years: Controlling Neuronal Migration and Maturation in the Mammalian Brain. Adv. Neurosci. 10.1155/2014/597395 [DOI] [Google Scholar]
- 2. Alcántara S., Ruiz M., D'Arcangelo G., Ezan F., de Lecea L., Curran T., Sotelo C., Soriano E. (1998) Regional and cellular patterns of reelin mRNA expression in the forebrain of the developing and adult mouse. J. Neurosci. 18, 7779–7799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. D'Arcangelo G., Miao G. G., Chen S. C., Soares H. D., Morgan J. I., Curran T. (1995) A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature 374, 719–723 [DOI] [PubMed] [Google Scholar]
- 4. Ogawa M., Miyata T., Nakajima K., Yagyu K., Seike M., Ikenaka K., Yamamoto H., Mikoshiba K. (1995) The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron 14, 899–912 [DOI] [PubMed] [Google Scholar]
- 5. Beffert U., Weeber E. J., Durudas A., Qiu S., Masiulis I., Sweatt J. D., Li W.-P., Adelmann G., Frotscher M., Hammer R. E., Herz J. (2005) Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor ApoER2. Neuron 47, 567–579 [DOI] [PubMed] [Google Scholar]
- 6. Borrell V., del Río J. A., Alcántara S., Derer M., Martínez A., D'Arcangelo G., Nakajima K., Mikoshiba K., Derer P., Curran T., Soriano E. (1999) Reelin regulates the development and synaptogenesis of the layer-specific entorhino-hippocampal connections. J. Neurosci. 19, 1345–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen Y., Beffert U., Ertunc M., Tang T. S., Kavalali E. T., Bezprozvanny I., Herz J. (2005) Reelin modulates NMDA receptor activity in cortical neurons. J. Neurosci. 25, 8209–8216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Del Río J. A., Heimrich B., Borrell V., Förster E., Drakew A., Alcántara S., Nakajima K., Miyata T., Ogawa M., Mikoshiba K., Derer P., Frotscher M., Soriano E. (1997) A role for Cajal-Retzius cells and reelin in the development of hippocampal connections. Nature 385, 70–74 [DOI] [PubMed] [Google Scholar]
- 9. Niu S., Renfro A., Quattrocchi C. C., Sheldon M., D'Arcangelo G. (2004) Reelin promotes hippocampal dendrite development through the VLDLR/ApoER2-Dab1 pathway. Neuron 41, 71–84 [DOI] [PubMed] [Google Scholar]
- 10. Niu S., Yabut O., D'Arcangelo G. (2008) The Reelin signaling pathway promotes dendritic spine development in hippocampal neurons. J. Neurosci. 28, 10339–10348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qiu S., Weeber E. J. (2007) Reelin signaling facilitates maturation of CA1 glutamatergic synapses. J. Neurophysiol. 97, 2312–2321 [DOI] [PubMed] [Google Scholar]
- 12. Weeber E. J., Beffert U., Jones C., Christian J. M., Forster E., Sweatt J. D., Herz J. (2002) Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J. Biol. Chem. 277, 39944–39952 [DOI] [PubMed] [Google Scholar]
- 13. Franco S. J., Martinez-Garay I., Gil-Sanz C., Harkins-Perry S. R., Müller U. (2011) Reelin regulates cadherin function via Dab1/Rap1 to control neuronal migration and lamination in the neocortex. Neuron 69, 482–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sinagra M., Verrier D., Frankova D., Korwek K. M., Blahos J., Weeber E. J., Manzoni O. J., Chavis P. (2005) Reelin, very-low-density lipoprotein receptor, and apolipoprotein E receptor 2 control somatic NMDA receptor composition during hippocampal maturation in vitro. J. Neurosci. 25, 6127–6136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. D'Arcangelo G., Homayouni R., Keshvara L., Rice D. S., Sheldon M., Curran T. (1999) Reelin is a ligand for lipoprotein receptors. Neuron 24, 471–479 [DOI] [PubMed] [Google Scholar]
- 16. Hiesberger T., Trommsdorff M., Howell B. W., Goffinet A. M., Mumby M. C., Cooper J. A., Herz J. (1999) Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of Disabled-1 and modulates Tau phosphorylation. Neuron 24, 481–489 [DOI] [PubMed] [Google Scholar]
- 17. Howell B. W., Hawkes R., Soriano P., Cooper J. A. (1997) Neuronal position in the developing brain is regulated by mouse disabled-1. Nature 389, 733–737 [DOI] [PubMed] [Google Scholar]
- 18. Sheldon M., Rice D. S., D'Arcangelo G., Yoneshima H., Nakajima K., Mikoshiba K., Howell B. W., Cooper J. A., Goldowitz D., Curran T. (1997) Scrambler and yotari disrupt the disabled gene and produce a reeler-like phenotype in mice. Nature 389, 730–733 [DOI] [PubMed] [Google Scholar]
- 19. Arnaud L., Ballif B. A., Förster E., Cooper J. A. (2003) Fyn tyrosine kinase is a critical regulator of disabled-1 during brain development. Curr. Biol. 13, 9–17 [DOI] [PubMed] [Google Scholar]
- 20. Bock H. H., Herz J. (2003) Reelin activates SRC family tyrosine kinases in neurons. Curr. Biol. 13, 18–26 [DOI] [PubMed] [Google Scholar]
- 21. Howell B. W., Herrick T. M., Cooper J. A. (1999) Reelin-induced tyrosine phosphorylation of Disabled 1 during neuronal positioning. Genes Dev. 13, 643–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Keshvara L., Benhayon D., Magdaleno S., Curran T. (2001) Identification of reelin-induced sites of tyrosyl phosphorylation on disabled 1. J. Biol. Chem. 276, 16008–16014 [DOI] [PubMed] [Google Scholar]
- 23. Ballif B. A., Arnaud L., Arthur W. T., Guris D., Imamoto A., Cooper J. A. (2004) Activation of a Dab1/CrkL/C3G/Rap1 pathway in Reelin-stimulated neurons. Curr. Biol. 14, 606–610 [DOI] [PubMed] [Google Scholar]
- 24. Gil-Sanz C., Franco S. J., Martinez-Garay I., Espinosa A., Harkins-Perry S., Müller U. (2013) Cajal-Retzius cells instruct neuronal migration by coincidence signaling between secreted and contact-dependent guidance cues. Neuron 79, 461–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jossin Y., Cooper J. A. (2011) Reelin, Rap1 and N-cadherin orient the migration of multipolar neurons in the developing neocortex. Nat. Neurosci. 14, 697–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park T. J., Curran T. (2008) Crk and Crk-like play essential overlapping roles downstream of disabled-1 in the Reelin pathway. J. Neurosci. 28, 13551–13562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ballif B. A., Arnaud L., Cooper J. A. (2003) Tyrosine phosphorylation of Disabled-1 is essential for Reelin-stimulated activation of Akt and Src family kinases. Brain Res. Mol. Brain Res. 117, 152–159 [DOI] [PubMed] [Google Scholar]
- 28. Beffert U., Morfini G., Bock H. H., Reyna H., Brady S. T., Herz J. (2002) Reelin-mediated signaling locally regulates protein kinase B/Akt and glycogen synthase kinase 3β. J. Biol. Chem. 277, 49958–49964 [DOI] [PubMed] [Google Scholar]
- 29. Jossin Y., Gui L., Goffinet A. M. (2007) Processing of Reelin by embryonic neurons is important for function in tissue but not in dissociated cultured neurons. J. Neurosci. 27, 4243–4252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Iafrati J., Orejarena M. J., Lassalle O., Bouamrane L., Chavis P. (2014) Reelin, an extracellular matrix protein linked to early onset psychiatric diseases, drives postnatal development of the prefrontal cortex via GluN2B-NMDARs and the mTOR pathway. Mol. Psychiatry 19, 417–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Trotter J., Lee G. H., Kazdoba T. M., Crowell B., Domogauer J., Mahoney H. M., Franco S. J., Muller U., Weeber E. J., D'Arcangelo G. (2013) Dab1 is required for synaptic plasticity and associative learning. J. Neurosci. 33, 15652–15668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jossin Y., Ignatova N., Hiesberger T., Herz J., Lambert de Rouvroit C., Goffinet A. M. (2004) The central fragment of Reelin, generated by proteolytic processing in vivo, is critical to its function during cortical plate development. J. Neurosci. 24, 514–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yasui N., Nogi T., Kitao T., Nakano Y., Hattori M., Takagi J. (2007) Structure of a receptor-binding fragment of reelin and mutational analysis reveal a recognition mechanism similar to endocytic receptors. Proc. Natl. Acad. Sci. U.S.A. 104, 9988–9993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kubo K., Mikoshiba K., Nakajima K. (2002) Secreted Reelin molecules form homodimers. Neurosci. Res. 43, 381–388 [DOI] [PubMed] [Google Scholar]
- 35. Utsunomiya-Tate N., Kubo K., Tate S., Kainosho M., Katayama E., Nakajima K., Mikoshiba K. (2000) Reelin molecules assemble together to form a large protein complex, which is inhibited by the function-blocking CR-50 antibody. Proc. Natl. Acad. Sci. U.S.A. 97, 9729–9734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nakano Y., Kohno T., Hibi T., Kohno S., Baba A., Mikoshiba K., Nakajima K., Hattori M. (2007) The extremely conserved C-terminal region of Reelin is not necessary for secretion but is required for efficient activation of downstream signaling. J. Biol. Chem. 282, 20544–20552 [DOI] [PubMed] [Google Scholar]
- 37. Koie M., Okumura K., Hisanaga A., Kamei T., Sasaki K., Deng M., Baba A., Kohno T., Hattori M. (2014) Cleavage within Reelin Repeat 3 Regulates the Duration and Range of the Signaling Activity of Reelin Protein. J. Biol. Chem. 289, 12922–12930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Comoletti D., Miller M. T., Jeffries C. M., Wilson J., Demeler B., Taylor P., Trewhella J., Nakagawa T. (2010) The macromolecular architecture of extracellular domain of alphaNRXN1: domain organization, flexibility, and insights into trans-synaptic disposition. Structure 18, 1044–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pfaffl M. W. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lambert de Rouvroit C., de Bergeyck V., Cortvrindt C., Bar I., Eeckhout Y., Goffinet A. M. (1999) Reelin, the extracellular matrix protein deficient in reeler mutant mice, is processed by a metalloproteinase. Exp. Neurol. 156, 214–217 [DOI] [PubMed] [Google Scholar]
- 41. Bock H. H., Jossin Y., Liu P., Förster E., May P., Goffinet A. M., Herz J. (2003) PI3-Kinase interacts with the adaptor protein Dab1 in response to Reelin signaling and is required for normal cortical lamination. J. Biol. Chem. 278, 38772–38779 [DOI] [PubMed] [Google Scholar]
- 42. Simó S., Pujadas L., Segura M. F., La Torre A., Del Río J. A., Ureña J. M., Comella J. X., Soriano E. (2007) Reelin induces the detachment of postnatal subventricular zone cells and the expression of the Egr-1 through Erk1/2 activation. Cereb Cortex 17, 294–303 [DOI] [PubMed] [Google Scholar]
- 43. Huang E. J., Reichardt L. F. (2003) Trk receptors: roles in neuronal signal transduction. Annu. Rev. Biochem. 72, 609–642 [DOI] [PubMed] [Google Scholar]
- 44. Marshall C. J. (1995) Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80, 179–185 [DOI] [PubMed] [Google Scholar]
- 45. Krueger D. D., Howell J. L., Hebert B. F., Olausson P., Taylor J. R., Nairn A. C. (2006) Assessment of cognitive function in the heterozygous reeler mouse. Psychopharmacology 189, 95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Larson J., Hoffman J. S., Guidotti A., Costa E. (2003) Olfactory discrimination learning deficit in heterozygous reeler mice. Brain Res. 971, 40–46 [DOI] [PubMed] [Google Scholar]
- 47. Qiu S., Korwek K. M., Pratt-Davis A. R., Peters M., Bergman M. Y., Weeber E. J. (2006) Cognitive disruption and altered hippocampus synaptic function in Reelin haploinsufficient mice. Neurobiol Learn Mem. 85, 228–242 [DOI] [PubMed] [Google Scholar]
- 48. Trommsdorff M., Borg J. P., Margolis B., Herz J. (1998) Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein. J. Biol. Chem. 273, 33556–33560 [DOI] [PubMed] [Google Scholar]
- 49. Herz J., Goldstein J. L., Strickland D. K., Ho Y. K., Brown M. S. (1991) 39-kDa protein modulates binding of ligands to low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. J. Biol. Chem. 266, 21232–21238 [PubMed] [Google Scholar]
- 50. Dalby K. N., Morrice N., Caudwell F. B., Avruch J., Cohen P. (1998) Identification of regulatory phosphorylation sites in mitogen-activated protein kinase (MAPK)-activated protein kinase-1a/p90rsk that are inducible by MAPK. J. Biol. Chem. 273, 1496–1505 [DOI] [PubMed] [Google Scholar]
- 51. Romeo Y., Zhang X., Roux P. P. (2012) Regulation and function of the RSK family of protein kinases. Biochem. J. 441, 553–569 [DOI] [PubMed] [Google Scholar]
- 52. Plath N., Ohana O., Dammermann B., Errington M. L., Schmitz D., Gross C., Mao X., Engelsberg A., Mahlke C., Welzl H., Kobalz U., Stawrakakis A., Fernandez E., Waltereit R., Bick-Sander A., Therstappen E., Cooke S. F., Blanquet V., Wurst W., Salmen B., Bösl M. R., Lipp H. P., Grant S. G., Bliss T. V., Wolfer D. P., Kuhl D. (2006) Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron 52, 437–444 [DOI] [PubMed] [Google Scholar]
- 53. Rodríguez J. J., Davies H. A., Silva A. T., De Souza I. E., Peddie C. J., Colyer F. M., Lancashire C. L., Fine A., Errington M. L., Bliss T. V., Stewart M. G. (2005) Long-term potentiation in the rat dentate gyrus is associated with enhanced Arc/Arg3.1 protein expression in spines, dendrites and glia. Eur. J. Neurosci. 21, 2384–2396 [DOI] [PubMed] [Google Scholar]
- 54. Chen R. H., Abate C., Blenis J. (1993) Phosphorylation of the c-Fos transrepression domain by mitogen-activated protein kinase and 90-kDa ribosomal S6 kinase. Proc. Natl. Acad. Sci. U.S.A. 90, 10952–10956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Doehn U., Hauge C., Frank S. R., Jensen C. J., Duda K., Nielsen J. V., Cohen M. S., Johansen J. V., Winther B. R., Lund L. R., Winther O., Taunton J., Hansen S. H., Frödin M. (2009) RSK is a principal effector of the RAS-ERK pathway for eliciting a coordinate promotile/invasive gene program and phenotype in epithelial cells. Mol. Cell 35, 511–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. O'Donnell A., Odrowaz Z., Sharrocks A. D. (2012) Immediate-early gene activation by the MAPK pathways: what do and don't we know? Biochem. Soc. Trans. 40, 58–66 [DOI] [PubMed] [Google Scholar]
- 57. Dulabon L., Olson E. C., Taglienti M. G., Eisenhuth S., McGrath B., Walsh C. A., Kreidberg J. A., Anton E. S. (2000) Reelin binds α3β1 integrin and inhibits neuronal migration. Neuron 27, 33–44 [DOI] [PubMed] [Google Scholar]
- 58. Belvindrah R., Graus-Porta D., Goebbels S., Nave K. A., Müller U. (2007) Beta1 integrins in radial glia but not in migrating neurons are essential for the formation of cell layers in the cerebral cortex. J. Neurosci. 27, 13854–13865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Graus-Porta D., Blaess S., Senften M., Littlewood-Evans A., Damsky C., Huang Z., Orban P., Klein R., Schittny J. C., Müller U. (2001) Beta1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron 31, 367–379 [DOI] [PubMed] [Google Scholar]
- 60. Förster E., Tielsch A., Saum B., Weiss K. H., Johanssen C., Graus-Porta D., Müller U., Frotscher M. (2002) Reelin, Disabled 1, and β1 integrins are required for the formation of the radial glial scaffold in the hippocampus. Proc. Natl. Acad. Sci. U.S.A. 99, 13178–13183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Senzaki K., Ogawa M., Yagi T. (1999) Proteins of the CNR family are multiple receptors for Reelin. Cell 99, 635–647 [DOI] [PubMed] [Google Scholar]
- 62. Sentürk A., Pfennig S., Weiss A., Burk K., Acker-Palmer A. (2011) Ephrin Bs are essential components of the Reelin pathway to regulate neuronal migration. Nature 472, 356–360 [DOI] [PubMed] [Google Scholar]
- 63. Catchpole T., Henkemeyer M. (2011) EphB2 tyrosine kinase-dependent forward signaling in migration of neuronal progenitors that populate and form a distinct region of the dentate niche. J. Neurosci. 31, 11472–11483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Stritt C., Knöll B. (2010) Serum response factor regulates hippocampal lamination and dendrite development and is connected with reelin signaling. Mol. Cell. Biol. 30, 1828–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Orban P. C., Chapman P. F., Brambilla R. (1999) Is the Ras-MAPK signalling pathway necessary for long-term memory formation? Trends Neurosci. 22, 38–44 [DOI] [PubMed] [Google Scholar]
- 66. Sweatt J. D. (2004) Mitogen-activated protein kinases in synaptic plasticity and memory. Curr. Opin. Neurobiol. 14, 311–317 [DOI] [PubMed] [Google Scholar]
- 67. Curtis J., Finkbeiner S. (1999) Sending signals from the synapse to the nucleus: possible roles for CaMK, Ras/ERK, and SAPK pathways in the regulation of synaptic plasticity and neuronal growth. J. Neurosci. Res. 58, 88–95 [PubMed] [Google Scholar]
- 68. Bouché E., Romero-Ortega M. I., Henkemeyer M., Catchpole T., Leemhuis J., Frotscher M., May P., Herz J., Bock H. H. (2013) Reelin induces EphB activation. Cell Res. 23, 473–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.