Abstract
Mechanisms that may underlie age and sex differences in the pharmacological effects of cannabinoids are relatively unexplored. The purpose of the present study was to determine whether sex differences in metabolism of Δ9-tetrahydrocannabinol (THC), similar to those observed previously in adult rats, also occurred in adolescent rats and might contribute to age and sex differences in its in vivo pharmacology. Male and female adolescent rats were exposed to THC acutely or repeatedly for 10 days. Subsequently, some of the rats were sacrificed and blood and brain levels of THC and one of its metabolites, 11-hydrox-Δ9-THC (11-OH-THC), were measured. Other rats were evaluated in a battery of in vivo tests that are sensitive to cannabinoids. Concentrations of 11-OH-THC in the brains of female adult and adolescent rats exceeded those observed in male conspecifics, particularly after repeated THC administration. In contrast, brain levels of THC did not differ between the sexes. In vivo, acute THC produced dose-related hypothermia, catalepsy and suppression of locomotion in adolescent rats of both sexes, with tolerance developing after repeated administration. With a minor exception, sex differences in THC’s effects in the in vivo assays were not apparent. Together with previous findings, the present results suggest that sex differences in pharmacokinetics cannot fully explain the patterns of sex differences (and lack of sex differences) in cannabinoid effects across behaviors. Hormonal and/or pharmacodynamic factors are also likely to play a role.
Keywords: adolescence, cannabinoids, in vivo pharmacology, metabolism, pharmacokinetics, rats, sex differences, delta-9-tetrahydrocannabinol, tolerance
Marijuana is one of the most commonly used illicit substances during adolescence. Amidst recent policy debates on medical marijuana and legalization of recreational marijuana, teen perception of marijuana’s dangerousness has decreased and its recreational use has correspondingly increased in this age group [1]. Despite their positive perceptions, however, adolescents may be at increased risk of adverse effects from marijuana, as extant research suggests that age of onset is important factor in determination of its short- and long-term effects [2,3,4,5,6]. Given that human users are self-selected, investigation of the mechanism(s) underlying these age effects has relied on animal models.
Like humans, rodents and other mammals undergo physical and behavioral changes around the time of puberty, which occur in the rat from approximately postnatal (PN) day 28 to 42 [7]. As in humans, age differences in cannabinoid pharmacology have been demonstrated in rodents, with adolescent rodents tending to show greater sensitivity to the behaviorally disruptive effects of cannabinoids and more long-term negative effects on brain and behavior [8,9,10,11]. In some studies, sex differences were also evident [12]. Research into the mechanism(s) of these differences is still in its infancy. The purpose of this study was to determine whether sex differences in THC metabolism, similar to those observed in adult rats [13,14], also occur in adolescent rats and might contribute to age and sex differences in cannabinoid in vivo pharmacology.
Methods
Subjects
Male and female Long-Evans rats were ordered from a commercial breeder (Harlan, Dublin, VA) as juveniles aged PN 22–25 or as adults (> PN65). Upon arrival, rats were housed in clear plastic cages in same-sex pairs and allowed at least 3 days to habituate to the vivarium environment. The vivarium was temperature-controlled (20–22°C) with a 12-hour light-dark cycle (lights on at 7 a.m.). Throughout the experiment, all rats had free access to food and water. The studies reported in this manuscript were carried out in accordance with guidelines published in the guide for the care and use of laboratory animals and were approved by the VCU IACUC.
Drugs
Δ9-Tetrahydrocannabinol (THC; National Institute on Drug Abuse, Bethesda, MD) was mixed in a vehicle of absolute ethanol, Emulphor-620 (Rhone-Poulenc, Inc., Princeton, NJ), and saline in a ratio of 1:1:18. All injections were administered at a volume of 1 ml/kg, with the exception that THC doses of 100 and 300 mg/kg were volume-adjusted from a 50 mg/ml solution.
Overall Experimental Design
This study involved three cohorts of rats, each containing male and female rats and each divided into treatment conditions. Rats in all cohorts were injected twice daily with vehicle or with THC for 9.5 days. Rats in the first cohort were adolescent during dosing and testing. On day 11, these rats were injected with cumulative doses of THC and were tested repeatedly in a battery of in vivo tests. Rats in the second cohort also were adolescent during dosing and testing. On day 11, these rats were injected with 10 mg/kg THC and were sacrificed 2 h later. Subsequently, concentrations of THC and one of its major metabolites were measured in the brain and blood of these rats. Rats in the third cohort were adult during dosing and testing. On day 11, these rats received identical treatment as the adolescent rats in the second cohort.
In Vivo Procedure
Male and female adolescent rats were randomly assigned to receive repeated dosing with either vehicle or THC. Beginning the morning of PN30, each rat was weighed and then received s.c. injections of vehicle or 10 mg/kg THC twice daily for 9 days and once in the morning on day 10 (PN39). Other than handling necessary for weighing, injecting and general cage maintenance, rats remained undisturbed in their home cages in the vivarium during the dosing regimen. On PN40, rats were assessed in a battery of three in vivo assays: locomotor activity, rectal temperature and an elevated bar test of catalepsy. At least one h before the start of the test battery, rats were transported to the laboratory and baseline temperature was measured. Immediately afterwards, rats began a cumulative dosing and testing procedure. First, they were injected i.p. with vehicle. Twenty min later each rat was placed in a locomotor chamber (Lafayette Instruments, Lafayette, IN) for 5 min and the total number of photocell beam breaks was recorded. Upon removal, temperature was measured again and change from baseline was calculated. Then, the front paws of the rat were placed on an elevated bar 30 min post-injection. The total amount of time (in s) that both paws remained in contact with the bar during a 5-min session was recorded and coverted to a percentage. If the rat voluntarily removed its paws from the bar 10 times, the session was stopped and amount of time on bar was recorded as 0. After the bar test (35 min after vehicle injection), each rat was injected i.p. with 10 mg/kg THC. The testing regimen described above was repeated 20 min later. Subsequently, each rat received additional doses of 20, 70 and 200 mg/kg THC (cumulative doses of 30, 100, and 300 mg/kg) and was re-tested following an identical procedure. Inter-dose interval was 35 min and completion of the entire cumulative dose-effect curve required 175 min (20 min pre-session injection interval and 15 min for testing = 35 min per dose X 5 doses = 175 min). Doses were based upon those used in a previous study with discrete (vs. cumulative) doses [11].
Quantification of Brain and Blood Levels
Separate male and female adolescent and adult rats were used for quantification of brain and blood levels of THC and a prominent psychoactive metabolite, 11-hydrox-Δ9-THC (11-OH-THC). The repeated dosing procedure with vehicle or 10 mg/kg THC was identical to that described above for adolescent rats in the in vivo study except that rats in this part of the study were sacrificed 2 h after an acute injection of 10 mg/kg THC (vehicle-treated rats) or 24 hours after last repeated injection of 10 mg/kg THC (i.e., day 11, at approximately the same time of day as the other rats were tested). Immediately after rats were killed by decapitation, blood was collected in heparinized tubes and chilled on wet ice. Subsequently, analytical measurements were taken between 30 and 40 min post mortem. Whole brains were rapidly dissected out on wet ice placed in a large cryovials and immediately snap frozen in liquid nitrogen and stored at −80°C until use. Extraction ofTHC and 11- OH-THC was carried out via a method previously described [15], and quantification was conducted via LC–MS as per [16].
Data Analysis
Mean (± SEM) values for each of the three measures were calculated across dose and time for each sex separately. For each dependent measure, separate mixed factorial ANOVAs (sex X cumulative dose X repeated treatment condition) were performed. Mean (± SEM) concentrations of THC and 11-OH-THC in blood and in brain were calculated separately for each sex and each chronic treatment condition. Separate factorial ANOVAs (sex X repeated treatment condition) were performed for THC and 11-OH-THC in blood and brain. Significant ANOVAs were further analyzed through the use of Tukey-Kramer post hoc tests (α=0.05).
Results
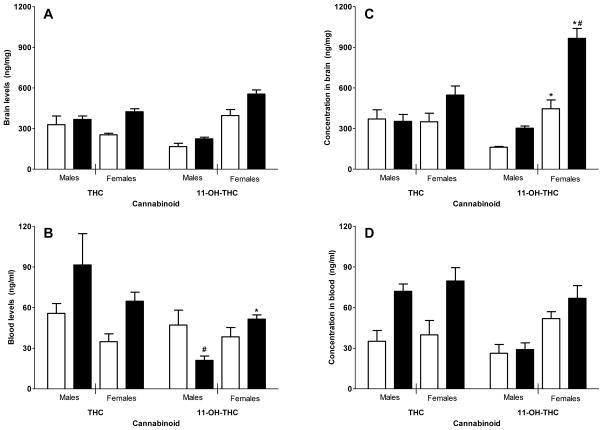
Figure 1 shows brain and blood levels of THC and 11-OH-THC after acute and repeated dosing with THC in adult and adolescent rats. In adult rats, brain levels of THC were significantly higher after repeated treatment than after acute treatment [Fig. 1, panel A; main effect of treatment: F(1,20)=7.94, p<0.05]. Brain levels of 11-OH-THC were significantly higher for adult females than for adult males [Fig. 1, panel A; main effect of sex: F(1,22)=96.15, p<0.05]. In addition, repeated treatment with THC produced higher brain levels of 11-OH-THC than did acute treatment [Fig. 1, panel A; main effect of treatment: F(1,22)=14.30, p<0.05]. Blood levels of 11-OH-THC were also significantly elevated in adult females than in age-matched males, but only after repeated treatment with THC [Fig. 1, panel B; sex X treatment: F(1,26)=9.93, p<0.05]. In male adults, blood levels of 11-OH-THC were significantly lower after repeated THC treatment than after acute treatment [Fig. 1, panel B; sex X treatment: F(1,26)=9.93, p<0.05]. In adult rats of both sexes, THC levels in the blood were significantly greater after repeated administration than after acute [Fig. 1, panel B; main effect of treatment: F(1,24)=6.39, p<0.05].
Figure 1.
Concentrations of THC and 11-OH-THC in brains (top panels) and blood (bottom panels) following acute or repeated dosing with 10 mg/kg Δ9-THC (unfilled and filled bars, respectively) in adult (> PN65; left panels) and adolescent (PN40; right panels) male and female Long-Evans rats. Bars represent the mean (± SEM) of data from the 5–8 rats. Asterisk (*) indicates significant interaction and sex difference between sexes for the same treatment condition. Number sign (#) indicates significant interaction and difference between treatments for the same sex. In addition to interaction effects indicated on the figure panels, the following main effects were significant: in panel A, significant main effects for treatment for THC and for 11-OH-THC, with repeated treatment resulting in higher concentrations than acute treatment. Also, in panel A, significant main effect for sex in 11-OH-THC concentrations showed greater brain levels of 11-OH-THC in females than in males. In panel B, significant main effect of treatment for THC indicated greater concentrations of THC in the blood of rats treated repeatedly with THC than in those treated acutely. In panel D, significant main effect of treatment for THC shows repeated treatment with THC results in more THC in the blood than acute treatment with THC. Also, in panel D, significant main effect of sex shows increased concentrations of 11-OH-THC in the blood of female (vs. male) rats. All differences were significant at p < 0.05.
In adolescent rats, brain levels of THC did not differ across sex nor did they differ significantly across duration of treatment (acute or repeated) [Fig. 1, panel C]. In contrast, brain levels of 11-OH-THC were significantly higher for adolescent females than adolescent males after acute and after repeated treatment with THC and were higher still for adolescent females with repeated treatment than with acute treatment [Fig. 1, panel C; sex X treatment condition: F(1,22)=13.91, p<0.05]. Blood levels of 11-OH-THC were also significantly elevated in adolescent females than in age-matched males, regardless of prior acute or repeated treatment [Fig. 1, panel D; main effect of sex: F(1,22)=23.36, p<0.05]. In adolescent rats of both sexes, THC levels in the blood were significantly greater after repeated administration than after acute [Fig. 1, panel D; main effect of treatment: F(1,23)=17.41, p<0.05].
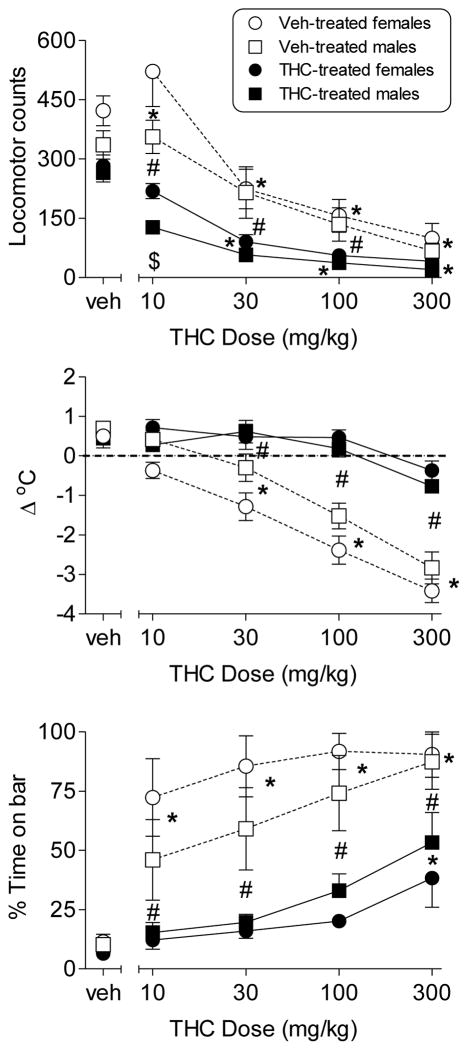
Figure 2 shows the effects of cumulative dosing with THC after twice daily injections of vehicle or 10 mg/kg THC for 9.5 days. Following repeated dosing with vehicle, female and male adolescent rats exhibited dose-dependent cannabimimetic effects. At cumulative doses of 30 mg/kg THC and higher, significant suppression of locomotor activity [Fig. 2, top panel; dose X treatment: F(4,80)=8.82, p<0.05] and hypothermia [Fig. 2, middle panel; dose X treatment: F(4,80)=37.5, p<0.05] were observed in rats of both sexes. At cumulative doses of 10 mg/kg THC and higher, significant catalepsy [Fig. 2, bottom panel; dose X treatment: F(4,80)=8.69, p<0.05] was observed in rats of both sexes. In contrast, after repeated dosing, THC was less potent in producing hypothermia and catalepsy in adolescent rats. In these THC-treated rats, THC did not produce hypothermia (Fig. 2, middle panel) and significantly increased catalepsy only at the 300 mg/kg dose (Fig. 2, bottom panel); dose X treatment: F(4,80)=8.69, p<0.05]. In addition, for both measures and across sexes, THC effects were significantly lesser in magnitude in the rats exposed to repeated THC versus those injected repeatedly with vehicle. Locomotor activity was significantly suppressed (compared to corresponding vehicle dose) by all cumulative doses of THC in the THC-treated rats [Fig. 2, top panel; dose X treatment: F(4,80)=8.82, p<0.05]. With one exception, significant sex differences in the effects of THC, acutely or after repeated administration, did not occur. The one exception was the significantly increased activity of female rats (compared to male rats) at the 10 mg/kg dose of THC [Fig. 2, top panel; sex X dose: F(4,80)=2.85, p<0.05].
Figure 2.
Effects of cumulative doses of THC on locomotor activity (top panel), change in rectal temperature (middle panel), and catalepsy (bottom panel) in male (squares) and female (circles) adolescent rats (PN40) following 9.5 days of twice daily treatment with vehicle (unfilled symbols) or 10 mg/kg THC (filled symbols). The leftmost points represent values after the first cumulative injection (vehicle). Each point represents mean (± SEM) values for 6 rats. Asterisk (*) indicates significant dose X treatment interaction and post hoc difference of the specified treatment (acute or repeated) compared to the respective vehicle data (regardless of sex). Number sign (#) indicates significant dose X treatment interaction and post hoc difference between acute and repeated treatment at the specified dose (i.e., tolerance). Dollar sign ($) indicates signficant sex X dose interaction and post hoc difference between sexes at the indicated dose (regardless of treatment). All differences were significant at p < 0.05.
Discussion
Metabolism of THC in mammals occurs primarily through the liver cytochrome P450 system [17]. In adult female rats, 11-OH-THC has been identified as the primary metabolite whereas analysis of metabolites in adult male rats reveals metabolism to a wider variety of mostly inactive cannabinoids [13,18,19]. The present results confirm the previous finding of greater concentration of 11-OH-THC in the brains of adult female rats (compared to males) and extend the finding to repeated administration. Further, the present results demonstrate that female adolescent rats, even more than female adult rats, exhibit pronounced metabolism of THC to 11-OH-THC compared to their male conspecifics, particularly after repeated THC administration. In contrast, brain levels of THC did not differ between the sexes. Given that 11-OH-THC shares THC’s psychoactivity [20,21], these results suggest that in vivo cannabimimetic activity after THC exposure could conceivably be potentiated by its metabolite in female adolescents and thereby, could represent a mechanism to account for increased female sensitivity to cannabinoid effects that has been observed in some studies [12].
To investigate this possibility, adolescent rats were evaluated in vivo in assays that have been shown to be sensitive to cannabinoids [22]. In adolescent rats of both sexes, acute dosing with THC produced dose-related hypothermia, catalepsy and suppression of locomotion, as has been shown previously in adolescent and adult rodents [11]. Further, tolerance to the hypothermic and cataleptic effects occurred following repeated dosing with THC in both sexes, as has also been shown previously [11]. Although the degree of tolerance was similar across sexes, two caveats prevent definitive conclusion of no sex differences. The first is that the THC dose-effect curves after repeated dosing with THC were flattened, suggesting that a floor effect may have interfered with the ability to see differences. The second issue is that the same dosing regimen of THC was used in both sexes to induce tolerance. Previous work with opioids has suggested that differences in the magnitude of tolerance may be altered by the use of the same tolerance induction dose in individuals that exhibit acute pharmacological differences [23]. Since female rats have shown greater sensitivity to some of THC’s pharmacological effects [12,21], treating them with the same tolerance induction regimen as males may have resulted in an effectively higher dose regimen, which could have, in turn, affected the degree of tolerance.
Despite these caveats, however, sex differences in THC’s effects in the in vivo assays were not apparent, with the exception of greater sensitivity of female (vs. male) adolescents to the locomotor stimulatory effects of a single dose of THC (10 mg/kg). Sex differences in the effects of THC in these assays were also not observed in adolescent rats during a previous study in which doses were administered discretely rather than cumulatively [11]. The lack of differences in vivo contrasts sharply with the large sex differences in metabolism. This disparity suggests that THC’s effects in these assays are not enhanced by its metabolism to 11-OH-THC in adolescent female rats. Together with previous findings, the present results suggest that sex differences in pharmacokinetics cannot fully explain the patterns of sex differences (and lack of sex differences) in cannabinoid effects across behaviors in adolescent and adult rodents. Hormonal and/or pharmacodynamic factors are also likely to play a role.
Highlights.
Mechanisms for sex differences in cannabinoid pharmacology are not well-studied.
Sex differences in Δ9-tetrahydrocannabinol (THC) metabolism were examined.
Predominant metabolism of THC in female adolescent and adult rats is to 11-OH-THC.
Sex differences in brain THC levels were not observed.
Despite sex differences in metabolism, in vivo differences were not seen.
Acknowledgments
Research supported by National Institute on Drug Abuse grant DA-016644. The authors thank Mary O’Connell, Justin Poklis, and Mary Tokarz for their excellent technical assistance in the conduct of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future national results on drug use: 1975–2013: Overview, Key Findings on Adolescent Drug Use. Ann Arbor: Institute for Social Research; Ann Arbor: The University of Michigan; 2014. [Google Scholar]
- 2.Battistella G, Fornari E, Annoni JM, Chtioui H, Dao K, Fabritius M, Favrat B, Mall JF, Maeder P, Giroud C. Long-term effects of cannabis on brain structure. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.67. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brook JS, Stimmel MA, Zhang C, Brook DW. The association between earlier marijuana use and subsequent academic achievement and health problems: a longitudinal study. Am J Addict. 2008;17:155–160. doi: 10.1080/10550490701860930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang L, Yakupov R, Cloak C, Ernst T. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain. 2006;129:1096–1112. doi: 10.1093/brain/awl064. [DOI] [PubMed] [Google Scholar]
- 5.Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, Gigerenzer G, Hoehe MR. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology. 1999;142:295–301. doi: 10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- 6.Jacobus J, Bava S, Cohen-Zion M, Mahmood O, Tapert ST. Functional consequences of marijuana use in adolescents. Pharmacol Biochem Behav. 2009;92:559–565. doi: 10.1016/j.pbb.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 8.Quinn HR, Matsumoto I, Callaghan PD, Long LE, Arnold JC, Gunasekaran N, Thompson MR, Dawson B, Mallet PE, Kashem MA, Matsuda-Matsumoto H, Iwazaki T, McGregor IS. Adolescent rats find repeated delta(9)-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology. 2008;33:1113–1126. doi: 10.1038/sj.npp.1301475. [DOI] [PubMed] [Google Scholar]
- 9.Rubino T, Vigano D, Realini N, Guidali C, Braida D, Capurro V, Castiglioni C, Cherubino F, Romualdi P, Candeletti S, Sala M, Parolaro D. Chronic delta(9)-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: Behavioral and biochemical correlates. Neuropsychopharmacology. 2008;33:2760–2771. doi: 10.1038/sj.npp.1301664. [DOI] [PubMed] [Google Scholar]
- 10.Schneider M, Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology. 2003;28:1760–1769. doi: 10.1038/sj.npp.1300225. [DOI] [PubMed] [Google Scholar]
- 11.Wiley JL, O’Connell MM, Tokarz ME, Wright MJ., Jr Pharmacological effects of acute and repeated administration of delta(9)-tetrahydrocannabinol in adolescent and adult rats. J Pharmacol Exp Ther. 2007;320:1097–1105. doi: 10.1124/jpet.106.108126. [DOI] [PubMed] [Google Scholar]
- 12.Craft RM, Marusich JA, Wiley JL. Sex differences in cannabinoid pharmacology: A reflection of differences in the endocannabinoid system? Life Sci. 2013;92:476–481. doi: 10.1016/j.lfs.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narimatsu S, Watanabe K, Yamamoto I, Yoshimura H. Sex difference in the oxidative metabolism of Δ9-tetrahydrocannabinol in the rat. Biochem Pharmacol. 1991;41:1187–1194. doi: 10.1016/0006-2952(91)90657-q. [DOI] [PubMed] [Google Scholar]
- 14.Tseng AH, Harding JW, Craft RM. Pharmacokinetic factors in sex differences in Δ9- tetrahydrocannabinol-induced behavioral effects in rats. Behav Brain Res. 2004;154:77–83. doi: 10.1016/j.bbr.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 15.Foltz RL, McGinnis KM, Chinn DM. Quantitative measurement of delta 9-tetrahydrocannabinol and two major metabolites in physiological specimens using capillary column gas chromatography negative ion chemical ionization mass spectrometry. Biomed Mass Spectrom. 1983;10:316–323. doi: 10.1002/bms.1200100503. [DOI] [PubMed] [Google Scholar]
- 16.Wiley JL, Jones AR, Wright MJ., Jr Exposure to a high-fat diet decreases sensitivity to Δ9-tetrahydrocannabinol-induced motor effects in female rats. Neuropharmacology. 2011;60:274–283. doi: 10.1016/j.neuropharm.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agurell S, Halldin M, Lindgren JE, Ohlsson A, Widman M, Gillespie H, Hollister L. Pharmacokinetics and metabolism of Δ1-tetrahydrocannabinol and other cannabinoids with an emphasis on man. Pharmacol Rev. 1986;38:21–43. [PubMed] [Google Scholar]
- 18.Narimatsu S, Watanabe K, Matsunaga T, Yamamoto I, Imaoka S, Funae Y, Yoshimura H. Cytochrome P-450 isozymes in metabolic activation of Δ9-tetrahydrocannabinol by rat liver microsomes. Drug Metab Dispos. 1990;18:943–948. [PubMed] [Google Scholar]
- 19.Narimatsu S, Watanabe K, Matsunaga T, Yamamoto I, Imaoka S, Funae Y, Yoshimura H. Cytochrome P-450 isozymes involved in the oxidative metabolism of delta-9-tetrahydrocannabinol by liver microsomes of adult female rats. Drug Metab Dispos. 1992;20:79–83. [PubMed] [Google Scholar]
- 20.Browne RG, Weissman A. Discriminative stimulus properties of delta 9- tetrahydrocannabinol: mechanistic studies. J Clin Pharmacol. 1981;21:227S–234S. doi: 10.1002/j.1552-4604.1981.tb02599.x. [DOI] [PubMed] [Google Scholar]
- 21.Tseng AH, Craft RM. Sex differences in antinociceptive and motoric effects of cannabinoids. Eur J Pharmacol. 2001;430:41–47. doi: 10.1016/s0014-2999(01)01267-5. [DOI] [PubMed] [Google Scholar]
- 22.Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, Johnson MR, Melvin LS, Mechoulam R, Ward SJ. Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol Biochem Behav. 1991;40:471–478. doi: 10.1016/0091-3057(91)90349-7. [DOI] [PubMed] [Google Scholar]
- 23.Barrett AC, Cook CD, Terner JM, Craft RM, Picker MJ. Importance of sex and relative efficacy at the mu opioid receptor in the development of tolerance and cross-tolerance to the antinociceptive effects of opioids. Psychopharmacology. 2001;158:154–164. doi: 10.1007/s002130100821. [DOI] [PubMed] [Google Scholar]