Abstract
The discernment of resource quality is pertinent to many daily decisions faced by animals. Public information is a critical information source that promotes quality assessments, attained by monitoring others’ performance. Here we provide the first evidence, to our knowledge, that chimpanzees (Pan troglodytes) use public information to guide resource selection. Thirty-two chimpanzees were presented with two simultaneous video demonstrations depicting a conspecific acquiring resources at a fast (resource-rich) or slow (resource-poor) rate. Subsequently, subjects selected the resource-rich site above chance expectation. As a comparison, we report evidence of public information use in young children. Investigation of public information use in primates is pertinent, as it can enhance foraging success and potentially facilitate payoff-biased social learning.
Keywords: public information, social information, social learning, social cognition
Social learning denotes behavior or learning that is altered according to other organisms’ presence, behavior, or behavioral products (Heyes, 1994). A large body of evidence indicates that many animal species are capable of social learning (Brown & Laland, 2003; Galef & Giraldeau, 2001; Reader & Biro, 2010; Reader & Laland, 2002), culminating in regional variation in behavior, suggestive of tradition or culture (Perry, 2011; van Schaik et al., 2003; Whiten et al., 1999). Wild chimpanzees, in particular, display one of the broadest cultural repertoires recorded, with geographical variation in food extraction and processing methods, as well as social behavior, thought to be underpinned by social learning rather than genetic or ecological factors alone (Whiten et al., 1999). Ancillary studies of captive chimpanzees support claims that social learning plays a role in regional behavioral variation in the wild (Horner, Proctor, Bonnie, Whiten, & de Waal, 2010; Whiten, Horner, & de Waal, 2005; Whiten et al., 2007). Indeed, both arbitrary behavioral traditions (Bonnie, Horner, Whiten, & de Waal, 2007) and foraging traditions (Horner, Whiten, Flynn, & de Waal, 2006) have been shown to emerge through social learning in this species.
A trend exists in the social learning literature to document how chimpanzees socially acquire foraging techniques (Hopper et al., 2007; Horner & Whiten, 2005) and, more recently, from whom they learn (Horner et al., 2010). In particular, focus has been given to the question of whether chimpanzees imitate (broadly defined as the copying of behavioral actions) or rely on other social learning processes (Hopper, Lambeth, Schapiro, & Whiten, 2008; Tennie, Call, & Tomasello, 2006), a question that remains a topic of debate (Tennie, Call, & Tomasello, 2009; Tennie, Call, & Tomasello, 2012). In addition, work on model-based, biased social learning has begun to document selective copying regarding to whom it is that chimpanzees attend and from whom they copy. Chimpanzees, for example, have been shown to preferentially copy dominant over low-ranking conspecifics, and selectively attend to the food-associated behavior of older or same-aged individuals (Biro et al., 2003; Horner et al., 2010; Kendal et al., 2013).
One area of interest that has received relatively little attention addresses whether social information influences chimpanzees’ decisions of where to forage and, in particular, whether the foraging successes of others act as cues to locating the most abundant food resources. When animals feed, they produce information, often inadvertently, through their performance, activities, and decisions, as well as in their by-products. This information can then be used by others as cues to resource locations (Danchin, Giraldeau, Valone, & Wagner, 2004). Theoretical modeling suggests that social learning (resulting in joining feeding conspecifics) outcompetes individual sampling in changing environments in which resources with high payoffs are associated with a high probability of samplers failing to find food (Arbilly, Motro, Feldman, & Lotem, 2011). Thus, for species that experience a variable food supply from which nutritional sources might be devoid, (e.g., seasonal fruits, Basabose, 2004; Watts, Potts, Lwanga, & Mitani, 2012), attending to foraging conspecifics may prove an adaptive strategy. Although the question of whether graded foraging performances cue resource quality judgments in primates remains understudied, evidence of the capacity to source and use social information to locate food resources has been documented in various primate species. The presence of a conspecific at one of two opaque food containers (local enhancement), for instance, can act as a social cue used by chimpanzees to locate a container baited with food (Itakura, Agnetta, Hare, & Tomasello, 1999). In a similar way, Tonkean macaques (Macaca tonkeana) have been shown to use both olfactory and visual residual signs, produced as a by-product of conspecific feeding, to locate distant food sources of the same type (Drapier, Chauvin, & Thierry, 2002).
Social cue use has been documented in all four great ape species (Pan troglodytes, Pan paniscus, Gorilla gorilla gorilla and Pongo pygmaeus abelii; Buttelmann, Call, & Tomasello, 2008). Specifically, various behavioral cues consistent with attempts to extract hidden food from one of two locations were used by subjects to infer the location of the hidden food sources. For example, a preference was displayed for baited containers, which the experimenter smelled and attempted to bite open, compared with those that were only smelled. It is of interest to note, Buttelmann and colleagues (2008) found that when subjects possessed personal knowledge of the absence of food in both containers, they selected at random, despite differential behavioral cues performed on the containers. Thus, social information use was dependent on subjects’ own knowledge states (i.e., personal information) and when personal and social information conflicted, a preference was displayed for the reliable, personal information; a “copy [only] when uncertain” strategy (Kendal, Coolen, & Laland, 2009; Kendal, Coolen, van Bergen, & Laland, 2005). More recently, chimpanzees have been shown to remember (inaccessible) locations at which they observed a human hide food items, and when eliciting the aid of a human to gain the hidden food items, they directed them first to items of high quality (Sayers & Menzel, 2012). Thus, the chimpanzees were able to store and use both personal information regarding resource quality and social information regarding the location of resources following a delay. What is novel about the current study is an investigation of whether chimpanzees use social cues to assess resource quality (public information sensu; Valone, 1989) and use this to guide their choices of resource locations.
Although studies have shown that primate species use social cues to locate hidden food (Buttelmann et al., 2008; Itakura et al., 1999), and that feeding conspecifics can socially facilitate other animals food consumption (Visalberghi & Addessi, 2000), little is known regarding whether primates are capable of discerning food abundance based on conspecifics’ foraging successes. One of the main daily decisions facing foragers is, of course, how to optimize energetic returns. When social information acts as a cue to resource quality it is termed “public information” (Valone, 1989). Public information, specifically, is a term derived from behavioral ecology, that, rather than referring to any information that is public (available to others), is confined to social information sourced from others’ performances conveying cues regarding quality (Valone, 1989; Valone, 2007; Valone & Templeton, 2002). This can include cues to abundant resources, successful breeding partners, habitats and breeding sites, and the quality of potential competitors (Valone, 2007). Public information use does not necessitate complex social learning processes; it can occur via local enhancement (Webster & Laland, 2012), feeding rate (Coolen, Bergen, Day, & Laland, 2003) and food-related collective commotion (Laidre, 2013).
Public information use has been assessed in the common marmoset (Callithrix jacchus; Voelkl & Huber, 2007). Marmoset pairs (demonstrator– observer) were presented with four pairs of opaque containers filled with wood chips, some of which were baited with food. The marmosets could forage simultaneously, with visual access to each other, but were separated by wire mesh. Equally, paired containers were positioned adjacent to one another but separated by mesh, so that resource sites matched for the marmoset pairs. The “demonstrator” marmoset was informed of food locations and thus, the “observer” marmoset could maximize its foraging success by synchronizing its search for food with that of the demonstrator. Contrary to expectation, however, the availability of this social information did not enhance foraging success.
In chimpanzees, auditory information can signal resource quality. Chimpanzees, upon locating food, produce rough-grunt vocalizations that differ according to the producer’s food preferences (Slocombe & Zuberbühler, 2006), offering important resourcequality information. Slocombe and Zuberbühler (2005) showed that a chimpanzee altered his foraging strategy according to playbacks of a high- versus low-quality food response, suggesting that rough grunts served as a social signal to resource quality. Overall, food-searching behavior was found to be prolonged and more thorough upon hearing rough grunts produced in response to high-quality food. Food searching, in addition, tended to be longer at those resource sites associated with the rough grunt played. Thus, rough grunts may constitute an important source of auditory public information.
In Experiment 1, we aimed to examine whether chimpanzees use visual public information— differential foraging behavior of a conspecific—to identify the most abundant food source in the absence of vocal signals. Public information is predicted to be widespread in nature, promoting greater accuracy in environmental assessments (Valone & Templeton, 2002). Yet, research into public information use has largely been confined to species of birds and fish (Valone, 2007). The study of public information in chimpanzees is vital for understanding which social information contributes to the daily decisions made by this species, including whether public information facilitates resource maximization. Public information use was recently reported in chimpanzees (Martin, Biro, & Matsuzawa, 2011); observers used models’ behavioral actions to solve a matching-to-sample task. However, as the copying of behavioral decisions was not confined to resource quality (as required for the strict use of public information sensu Valone, 1989) to date, whether chimpanzees discern patch profitability by monitoring the relative success of conspecifics is unknown.
We employed a variant of Coolen, Bergen’s et al. (2003) methodology to examine whether chimpanzees use graded information to assist them in their own foraging decisions, of conspecific foraging at food-rich and food-poor sites. Simultaneous videos of a conspecific acquiring resources at two locations, each differing in terms of the rate at which food was gained (food-rich vs. food-poor), were presented. Subsequently, observer chimpanzees were given access to the resource sites, and their selections recorded. Employing video-based social stimuli with chimpanzees (Hopper, Lambeth, & Schapiro, 2012) offers the advantage of presenting the same unfamiliar model at each foraging site, thus controlling for any model-based biases (Rendell et al., 2011). This is important, due to the established influence of social dynamics, age, and perhaps previous track record of success (Biro et al., 2003; Horner et al., 2010; Kendal et al., 2013), to whom it is that chimpanzees attend and from whom they learn. As bird and fish species use public information (Valone, 2007), and given chimpanzees’ sensitivity to behavioral cues in foraging situations, their discerning auditory food signals (Slocombe & Zuberbühler, 2005), and their ability to engage in observational learning (Martin et al., 2011), we predicted that chimpanzees would display the ability to use public information.
We were additionally interested in the ability of 5-year-old children (Homo sapiens) to use public information, as, to our knowledge, whether children use public information to discern reward quality has yet to be empirically investigated. In Experiment 2, we replicated the chimpanzee study with 5-year old children (Homo sapiens) using a similar methodology. This follows previous studies that have focused on the sociocognitive skills of both chimpanzees and children, finding that young children constitute an appropriate group with which chimpanzees can be compared (Dean, Kendal, Schapiro, Thierry, & Laland, 2012; Herrmann, Call, Hernández-Lloreda, Hare, & Tomasello, 2007; Horner & Whiten, 2005). Similar to chimpanzees, the feeding behavior of children shows susceptibility to social context. Children’s food preferences, for example, have been shown to alter in accordance with peer preferences (Birch, 1980a). Children’s food intake and preferences have also been documented to positively correlate with those of parents and other adults of the same subculture (Birch, 1980b; Orlet Fisher, Mitchell, Wright, & Birch, 2002), and the amount of food consumed has been shown to vary according to one’s own size and social partner size (Salvy, Romero, Paluch, & Epstein, 2007). Given the social influence on feeding behavior and that children readily respond to social information (Lyons, Damrosch, Lin, Macris, & Keil, 2011; Wood, Kendal, & Flynn, 2012), we predicted that children would use public information as a cue to resource quality.
Experiment 1: Chimpanzees
The purpose of this experiment was to establish whether individual chimpanzees would assess resource quality by monitoring the relative foraging success of a conspecific feeding at different rates (public information use).
Method
Subjects
Thirty-nine chimpanzees participated; three were discounted, as they did not interact with the resource boxes during a pretesting phase (see Procedure) and four were discounted due to inattention to the demonstrations. The remaining 32 chimpanzees (16 male) ranged in age from 15 to 44 years (M = 30). Following previous studies, a dominant, female (Hopper, Schapiro, Lambeth, & Brosnan, 2011) unfamiliar chimpanzee served as the demonstrator. Subjects were housed at the Michale E. Keeling Center for Comparative Medicine and Research (KCCMR) facility in Bastrop, Texas. The KCCMR is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. The chimpanzees were group-housed with access to enriched indoor and outdoor enclosures with climbing facilities. Subjects had participated in previous video social learning tasks (Hopper et al., 2012) and had past exposure to video for enrichment. No food or water deprivation was used during this study, which was approved by the Life Sciences Ethical Review Committee, Durham University, Durham, England, and the Institutional Animal Care and Use Committee of The University of Texas M. D. Anderson Cancer Center.
Video stimuli
Video demonstrations showed a model acquiring rewards (peanuts) at different rates (rich: approximately every 12 s, poor: approximately every 84 s; see Figure 1) from two boxes (21.5 H × 10W × 30 L cm). To achieve this, each box had a small hole situated at the back through which the food items were dispensed by the experimenter. The demonstrator could then retrieve the food items by reaching inside an opening at the front of the box. Thus, the peanuts themselves were barely visible, but the foraging/eating actions of the demonstrator were. The two boxes, resource-rich and resource-poor, were colored either yellow or black. To allow counterbalancing of the box color constituting the rich resource sites during the test sessions, four video demonstrations were captured (yellow-rich; black-poor; black-rich; yellow-poor, with the same demonstrator used in all demonstrations). To ensure that the demonstrator sourced individual peanuts at the predetermined rates, where appropriate video demonstrations were edited slightly using Picture Motion Browser (Sony) and Windows Live Movie Maker. Video editing consisted of cutting and/or looping subsections of the demonstrations. All recordings were captured with a Sony Handycam.
Figure 1.
Model retrieving rewards from the resource boxes (video-demonstration stills).
Design and procedure: Pretests
As neophobic reactions to novel objects can occur in chimpanzees, a habituation stage was performed to expose subjects to the resource boxes prior to running the experiment. Chimpanzees were given sequential, color-counterbalanced exposure to the baited resource boxes. Chimpanzees that did not retrieve a grape from both boxes during this session (N = 3) were eliminated from the study. This pretest identified subjects who lacked the motivation to participate and/or those who would fail to select a resource box in test sessions due to neophobic responses to the apparatus.
Color preference was assessed using a dichotomous preference paradigm (Hopper et al., 2011). In 10 successive, counterbalanced and unrewarded trials, the experimenter simultaneously held one cylindrical token (yellow/black) in each hand and chimpanzees selected one via gesture. No color preferences were observed (yellow token selections from 10 trials M = 4.81, SD = .90; binomials, all p > .05, N = 36).
Design and procedure: Experimental test
Chimpanzees were tested individually and voluntarily within their indoor compartments (circa 2.4 × 2.4 × 1.8 m3). Demonstrations were presented on two computer monitors (48.26 cm) on separate trolleys (85 H × 51 W × 51 L cm) located adjacent to one another (separated by roughly 40 cm). The two opaque boxes (yellow/black), from which the demonstrator retrieved resources, were positioned in front of the trolleys behind an occluding barrier, and positioned (left/right) to match the box color depicted in the corresponding video. The color (yellow/black) constituting the resource-rich patch and the side (left/right) presented were counterbalanced. All subjects received one trial only. Test sessions were video recorded.
Following the demonstrations, the resource boxes were simultaneously revealed by removal of the occluding barrier and pushed toward the subjects. The resource boxes were designed such that the observers could not see the food rewards inside until they had placed their hand inside the hole at the front. Resource selection was defined as the first resource box the subjects touched. The unselected box was then removed by the experimenter to prevent chimpanzees from gaining rewards from both boxes. Upon box selection, chimpanzees could retrieve the food item from their chosen box. To reduce food intake, and since only one trial was conducted with each subject, resource boxes were each baited with one banana piece only, irrespective of box quality. This also prevented potential olfactory cues arising from a large amount of food placed in one box only. Subjects were allocated up to 2 min to make their selection, after which the trial would be terminated and the subject discounted. In practice, all selections were made in less than 13 s and no individuals were discounted. Subjects’ attention (head orientation) to the videos was noted at 10-s intervals, and those (N = 4) not meeting a criterion of attention at ≥6 10-s intervals, were discounted.
Design and procedure: Statistical analysis
Due to the small sample size and dichotomous dependent variable, nonparametric statistics were used. First we investigated whether the number of resource-rich selections differed from chance (50%) using the binomial test. Mann–Whitney U tests were conducted to ascertain whether subject age, latency to box selection, and attention levels differed according to resource selection (rich/poor). Whether resource selection differed according to sex, the video sets viewed (yellow-rich/black-rich), or the sequential order of box presentation during the pretest habituation phase, was assessed using chi-square and Fisher’s exact (i.e., where contingency tables contained expected values of below five) tests. In addition, binomial tests were used to assess side and color biases in resource selections (chance = 50%).
Results
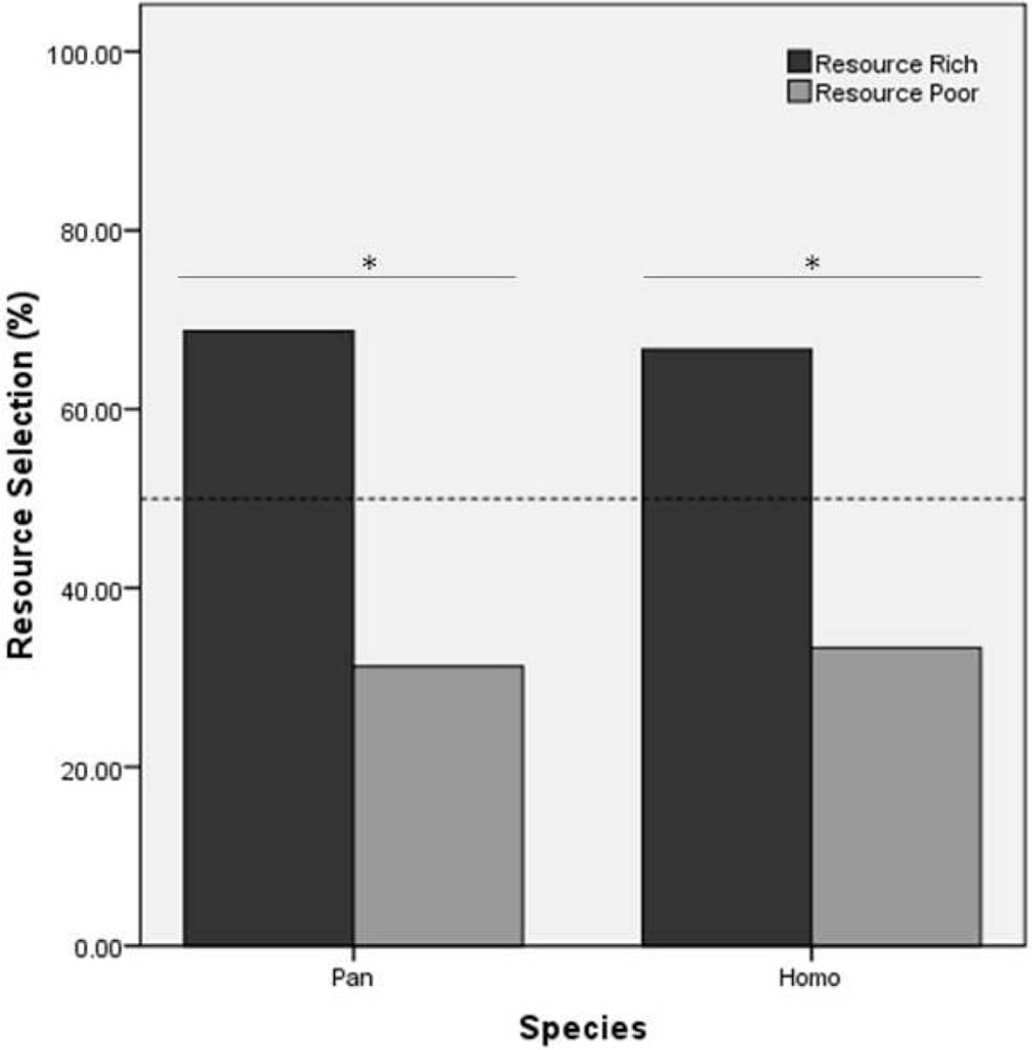
As predicted, the majority of (22 of the 32) chimpanzees selected the resource-rich box following presentation of the demonstrations (binomial, p = .03, one-tailed, 95% CI [.53, .84]; see Figure 2). The mean time taken to select a resource box was 4 s (SD = 3). No significant differences were observed between age, U = 74.50, N = 32, p = .15, sex, χ2(1) = 2.33, p = .25, latency to selection, U = 94.50, N = 32, p = .54, or attendance, U = 95.50, N = 32, p = .57, as a function of the resource box selected.
Figure 2.
Resource-rich and resource-poor selections (%) per species. Dotted line represents chance level, * = p < .05.
There was no significant difference in resource-box selection following the demonstration as a function of the box presented first during the box exposure pretest, χ2(1, 32) = 2.32, p = .25. Resource-box selections did not differ according to the different video sets needed to counterbalance color and resource richness (Fisher’s exact test: N = 32, p = 1. 00). Moreover, the chimpanzees displayed no side bias (binomial: N = 32, p = .38, left N = 13 and right N = 19) nor color bias (binomial: N = 32, p = .86, black N = 15 and yellow N = 17).
Experiment 2: Children
Experiment 1 showed that chimpanzees discerned resource quality from video demonstrations. We now turn to the question of whether children use public information to assess resource quality. The child study methodology was identical to the chimpanzee study except for changes, detailed below, to accommodate species differences.
Participants
Thirty-six 5-year-old children (17 male) were recruited from three primary schools in the North East of England. An individual girl, unfamiliar to the participants (aged 5 years), acted as the demonstrator via video for all children.
Video Stimuli
Video presentations were shorter in duration (1 min 40 s) than for the chimpanzees and, due to retention of the overall resource quantities presented (rich 15 vs. poor 3), the rate at which each reward was dispensed at the resource-rich location was increased (from every 12 to every 6 s; see Table 1). Stickers constituted the resource due to the ethical considerations of provisioning consumables. The boxes consisted of two opaque, hemisphere-shaped plastic containers (total surface area 763.41 cm2). As children displayed color preferences (Boyatzis & Varghese, 1994), the boxes were differentiated by pattern (large or small black squares). Upon retrieving stickers, the demonstrator placed them in an opaque cup. This prevented a stack of stickers from accumulating, which could have served as an additional cue for the children, relative to chimpanzees, in whose case rewards were immediately consumed by the demonstrator.
Table 1.
Time (Minutes, Seconds) at Which Single Rewards Were Dispensed During Demonstrations
| Pan | Homo | ||
|---|---|---|---|
| Resource-rich | Resource-poor | Resource-rich | Resource-poor |
| 0.05 | 0.05 | 0.06 | 0.06 |
| 0.17 | 0.12 | ||
| 0.29 | 0.18 | ||
| 0.41 | 0.24 | ||
| 0.53 | 0.30 | ||
| 1.05 | 0.36 | ||
| 1.17 | 0.42 | ||
| 1.29 | 1.29 | 0.48 | 0.48 |
| 1.41 | 0.54 | ||
| 1.53 | 1.00 | ||
| 2.05 | 1.06 | ||
| 2.17 | 1.12 | ||
| 2.29 | 1.18 | ||
| 2.41 | 1.24 | ||
| 2.53 | 2.53 | 1.30 | 1.30 |
Design and Procedure
Testing was conducted in a quiet room at each child’s school away from the rest of their class. Each child participated in one trial only. Participants were told by an experimenter (G. V.), “I would like you to watch videos of a girl getting stickers, and then after the videos you will get a chance to find stickers” and given verbal prompts (“are you watching the videos?” “can you see what the little girl is doing?”) if attention lapsed. It is noteworthy that the children were encouraged to attend to the videos by experimenter prompting, with no verbal prompts given to the chimpanzees. Following the videos, the occluding barrier was removed to reveal the resource boxes and children were instructed, “You can have a look in the boxes now.” Participants were allocated up to 1 min to make a resource selection, defined as the first box touched or gestured toward. The chosen box was then opened to retrieve the stickers. The number of baited stickers in the resource-rich and resource-poor boxes matched the number obtained by the demonstrator in the corresponding videos (15 and 3, respectively). As the data were derived from one trial only, the number of stickers gained did not influence the study results.
Results
As predicted, 24 of 36 children selected the resource-rich location, which is above that predicted by chance alone (binomial test: p = .03, one-tailed, 95% CI [.51, .82] see Figure 2). Average time to box selection was 6 s (SD = 5). Resource selection was not related to sex, χ2(1) = .22, p = .73, or box selection latency (U = 95.50, N = 36, p = .10). No side (binomial test; N = 36, p = .24) or box pattern preferences (binomial test; N = 36, p = .62) were observed. There was no significant difference in the species’ tendencies to choose the “rich” patch, χ2(1) = .03, p = 1.00.
Discussion
Chimpanzees (Pan troglodytes) and children (Homo sapiens) are capable of social learning (Horner et al., 2006). Numerous studies have documented that group-specific traditions occur in these species through differential copying of knowledgeable conspecifics (Flynn & Whiten, 2008; Whiten et al., 2005; Whiten et al., 2007). Such studies have tended to concentrate on the copying of behavioral methods, often using tools, of gaining a food reward that exhibits (novel) food-extractive behavior. Less is known about whether social information relating to differential food abundance guides primates’ subsequent foraging decisions. The ability to discriminate between resource qualities using public information allows profitable food sources to be identified and visited with potentially greater accuracy than if using personal information alone (Arbilly et al., 2011; Valone, 2007). Our results indicate that chimpanzees and 5-year-old children possess this ability, interestingly showing high concordance in public information use across species. Thus, in addition to attending to social cues to locate food sources (Buttelmann et al., 2008; Itakura et al., 1999), chimpanzees and children were able to select reward sources according to the graded acquisition (of food/stickers) performance of a conspecific. Children and chimpanzees thus performed at comparable levels despite methodological differences, which included verbal attention prompts for children but not chimpanzees, and the provisioning of stickers rather than consumables.
Foraging decisions rely on various cognitive skills. Route planning, cognitive maps, memory of food sources, travel time, competition for food and likelihood of patch depletion can all influence decisions of where to forage (Noser & Byrne, 2010). Much of this information is derived from personal experience; however, social foragers are afforded an additional information source derived from others’ activities (Dall, Giraldeau, Olsson, McNamara, & Stephens, 2005). Our results suggest that public information sourced from conspecific foraging success may, in addition to personal information (Beran, Evans, & Harris, 2008) and auditory signals (Slocombe & Zuberbühler, 2006), aid in locating quality resources in chimpanzees and hence constitute one more factor among many that could contribute to foraging decisions
The use of public information has many implications. Primates may optimize foraging efficiency through exploiting inadvertent social information manifested in the foraging activity of conspecifics (Arbilly et al., 2011). In the present study, the relative number of times or the rate at which the demonstrator reached inside each resource box to acquire reward items, and the subsequent consumption activity for chimpanzees, could constitute potential cues by which resource quality was determined. Future investigation would benefit from control conditions to isolate the cues utilized to discern resource abundance. The inclusion of consumption only and reward retrieval without consumption would prove beneficial conditions in this regard.
Public information use can allow patch estimation to occur without engaging in personal sampling (Coolen et al., 2003). Public information may therefore aid decisions of food approach through an assessment of whether food sources will support additional foragers without direct food contest. That is, use of public information could benefit foragers through conflict avoidance by allowing a predetermination of whether approach would likely result in conflict due to low-resource abundance versus safer approach to more abundant nonmonopolizable food sources. When public information is derived from successful dominant foragers, an ability to use it following the departure of that individual may prove beneficial for subordinate observers (McQuoid & Galef, 1992). Chimpanzees have been shown to remember, following a delay, locations they previously saw a human hide food and to “direct” a human helper to hidden food of high quality first (Sayers & Menzel, 2012). This, along with other numerous studies, show that chimpanzees are capable of delayed social information use (Bering, Bjorklund, & Ragan, 2000; Bjorklund, Yunger, Bering, & Ragan, 2002). It is worth noting, however, that where food is markedly limited, public information will be of little value, even after a delay, because due to depletion, food consumption depends upon who discovers it first (Giraldeau, Valone, & Templeton, 2002). In this context, reliance upon personal information would best serve the forager. Thus considerations of public and personal information use are pertinent to chimpanzees, a species in which fission–fusion dynamics are pronounced, as they allow assessment of resource distribution and abundance, factors that can underwrite party size (Aureli et al., 2008).
Public information has the potential to aid foraging activity through signaling patch depletion (Fraser, Ruxton, & Broom, 2006; Templeton & Giraldeau, 1995). It is of interest that chimpanzees and children selected the resource box associated with the demonstrator retrieving rewards at the fastest rate. This suggests that the faster feeding rates did not signal patch depletion. Because increased feeding rate can mark rapid food depletion, sustained high rates should signal food abundance and slower (or reducing) rates should indicate limited food supply. Finding that chimpanzees and children displayed a preference for the resource supporting rapid food retrieval is in line with reports that species are attracted to food sites at which the feeding rate is faster (Coolen et al., 2003; Coolen, Ward, Hart, & Laland, 2005). To investigate whether public information provides cues to patch depletion, it would be of interest to examine the influence of demonstrator-foraging success, varying success (x retrieval attempts with no food obtained) and the feeding rate (gradual reduction vs. increased rate of food obtained) in addition to utilizing real-time demonstrations.
Although chimpanzees in this study displayed a preference for the rich resource box, it remains unclear whether this finding would hold in a group context. Video footage of a foraging demonstrator, theoretically, could have alleviated any competitive foraging demands that would otherwise occur in more naturalistic group settings, including dominance factors (Emery Thompson, Muller, Kahlenberg, & Wrangham, 2010; Muller & Wrangham, 2004; Sapolsky, 1992). This scenario is beneficial for the establishment of whether chimpanzees can use public information, but nevertheless does not allow an assessment of whether they do use public information more generally in the wild (Boesch, 2007, 2008). In groups, factors such as the dominance rank of those already foraging, the number of foragers, food distribution (monopolizable or not) and species level foraging strategies (e.g., contest and/or scramble competition) will likely play a prominent role in foraging decisions (Murray, Eberly, & Pusey, 2006; Murray, Mane, & Pusey, 2007).
Moreover, in chimpanzees, foraging strategies also differ according to sex and reproductive status. Lactating females tend to visit fewer of the available high-value resources per day than do sexually receptive females and males, but stay at resource locations longer (Bates & Byrne, 2009). Males, in contrast, have been shown to use linear daily foraging paths, indicative of a strategy of combining foraging needs with territorial defense (Bates & Byrne, 2009). Accordingly, although chimpanzees in this study showed public information use, individual foraging strategies employed in the wild, including patch departures, are mediated by optimizing food intake, and other factors such as sex-specific needs. Thus, foraging decisions in this species represents a complex process that may not only rely on personal and public information, but one that is also variable according to individual needs.
To understand decision making in chimpanzees (and children) it is important to determine the information sources underpinning behavioral actions. In this study we demonstrated that public information derived from differential foraging success can influence subsequent foraging decisions. How human and nonhuman primates weigh personal and public information, especially when they conflict (Kendal et al., 2005), and how social dynamics (Coussi-Korbel & Fragaszy, 1995), such as dominance rank, influence public information use, represent further important questions. Moreover, the pertinence of public information, especially in species-displaying traditions (Laland & Galef, 2009), lies in its use enabling payoff assessments of resources without participating in personal sampling, which can be costly in terms time and energy losses (Valone, 2007). To this end, public information has the potential to facilitate informed payoff-biased copying decisions, whereby individuals adopt behaviors in proportion to their profitability. One aspect of import to cumulative culture, in which cultural traits and behaviors become more complex and efficient across generations such that a single individual could never invent the trait within its lifetime (Tennie et al., 2009), is recognizing when a behavioral option is a beneficial modification that should be incorporated into the existing cultural trait (Laland, 2004). Public information may promote rudimentary “copy if better strategies” (Schlag, 1998, 1999), allowing the “ratcheting up” (Tennie et al., 2009) of cultural traits (e.g., technology) over generations. If quality assessments—made through monitoring the relative payoffs gained by conspecifics, or one’s self, using different traits— encourage the social acquisition of beneficial trait modifications (e.g., food-processing techniques), these could have potential consequences for cultural evolution. Specifically, it is possible that cumulative culture, which is widely held to be responsible for humanity’s success (Kendal, Rendell, Pike, & Laland, 2009), depends upon use of payoff-biased social learning strategies. Whether public information use may promote selectivity in what is copied through facilitating such payoff-biased social learning, and whether use of such cultural transmission biases (Rendell et al., 2011) is instrumental in the observed cross-species distribution of cumulative culture (Dean et al., 2012), requires further investigation.
Acknowledgment
G. L. V. was funded by a Durham University health and social science interdisciplinary studentship. R. L. K was funded by a Royal Society Dorothy Hodgkin Fellowship, and R. L. K and E. G. F were supported by a Durham University Seedcorn Fund. S. J. S and S. P. L were supported by the United States Department of Health and Human Services, National Institutes of Health Grant NIH RR-15090. We thank Hartside Primary, Stanley Crook Primary, and Leadgate Infant and Nursery schools, Alina Kendal (demonstrator), staff at the Michale E. Keeling Center for Comparative Medicine and Research and Lydia Hopper for logistical support. We are grateful for the helpful comments of two reviewers, Richard Moore and Irene Pepperberg.
Contributor Information
Gill L. Vale, Centre for Coevolution of Biology & Culture, Department of Anthropology & Department of Psychology, Durham University, Durham, England
Emma G. Flynn, Department of Psychology, Durham University
Susan P. Lambeth, Department of Veterinary Sciences, The University of Texas M. D. Anderson Cancer Center
Steven J. Schapiro, Department of Veterinary Sciences, The University of Texas M. D. Anderson Cancer Center
Rachel L. Kendal, Department of Anthropology, Centre for Coevolution of Biology & Culture, Durham University
References
- Arbilly M, Motro U, Feldman MW, Lotem A. Evolution of social learning when high expected payoffs are associated with high risk of failure. Journal of the Royal Society Interface. 2011;8:1604–1615. doi: 10.1098/rsif.2011.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aureli F, Schaffner CM, Boesch C, Bearder SK, Call J, Chapman CA. Fission-Fusion Dynamics New Research Frameworks. Current Anthropology. 2008;49(4):627–654. [Google Scholar]
- Basabose AK. Fruit availability and chimpanzee party size at Kahuzi montane forest, Democratic Republic of Congo. Primates. 2004;45:211–219. doi: 10.1007/s10329-004-0087-7. [DOI] [PubMed] [Google Scholar]
- Bates L, Byrne R. Sex differences in the movement patterns of free-ranging chimpanzees (Pan troglodytes schweinfurthii): foraging and border checking. Behavioral Ecology and Sociobiology. 2009;64:247–255. [Google Scholar]
- Beran MJ, Evans TA, Harris EH. Perception of food amounts by chimpanzees based on the number, size, contour length and visibility of Items. Animal Behaviour. 2008;75:1793–1802. doi: 10.1016/j.anbehav.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bering JM, Bjorklund DF, Ragan P. Deferred imitation of object-related actions in human-reared juvenile chimpanzees and orangutans. Developmental Psychobiology. 2000;36:218–232. doi: 10.1002/(sici)1098-2302(200004)36:3<218::aid-dev5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Birch LL. Effects of peer models’ food choices and eating behaviors on preschoolers’ food preferences. Child Development. 1980a;51:489–496. [Google Scholar]
- Birch LL. The relationship between children’s food preferences and those of their parents. Journal of Nutrition Education. 1980b;12:14–18. [Google Scholar]
- Biro D, Inoue-Nakamura N, Tonooka R, Yamakoshi G, Sousa C, Matsuzawa T. Cultural innovation and transmission of tool use in wild chimpanzees: Evidence from field experiments. Animal Cognition. 2003;6:213–223. doi: 10.1007/s10071-003-0183-x. [DOI] [PubMed] [Google Scholar]
- Bjorklund DF, Yunger JL, Bering JM, Ragan P. The generalization of deferred imitation in enculturated chimpanzees (Pan troglodytes) Animal Cognition. 2002;5:49–58. doi: 10.1007/s10071-001-0124-5. [DOI] [PubMed] [Google Scholar]
- Boesch C. What makes us human (Homo sapiens)? The challenge of cognitive cross-species comparison. Journal of Comparative Psychology. 2007;121:227–240. doi: 10.1037/0735-7036.121.3.227. [DOI] [PubMed] [Google Scholar]
- Boesch C. Taking development and ecology seriously when comparing cognition: Reply to tomasello and call (2008) Journal of Comparative Psychology. 2008;122:453–455. doi: 10.1037/0735-7036.122.4.453. [DOI] [PubMed] [Google Scholar]
- Bonnie KE, Horner V, Whiten A, de Waal FBM. Spread of arbitrary conventions among chimpanzees: A controlled experiment. Proceedings of the Royal Society: B. Biological Sciences. 2007;274:367–372. doi: 10.1098/rspb.2006.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyatzis CJ, Varghese R. Childrens emotional associations with colors. The Journal of Genetic Psychology: Research and Theory on Human Development. 1994;155:77–85. doi: 10.1080/00221325.1994.9914760. [DOI] [PubMed] [Google Scholar]
- Brown C, Laland KN. Social learning in fishes: A review. Fish and Fisheries. 2003;4:280–288. [Google Scholar]
- Buttelmann D, Call J, Tomasello M. Behavioral cues that great apes use to forage for hidden food. Animal Cognition. 2008;11:117–128. doi: 10.1007/s10071-007-0095-2. [DOI] [PubMed] [Google Scholar]
- Coolen I, Bergen YV, Day RL, Laland KN. Species difference in adaptive use of public information in sticklebacks. Proceedings of the Royal Society of London: Series B. Biological Sciences. 2003;270:2413–2419. doi: 10.1098/rspb.2003.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolen I, Ward AJW, Hart PJB, Laland KN. Foraging nine-spined sticklebacks prefer to rely on public information over simpler social cues. Behavioral Ecology. 2005;16:865–870. [Google Scholar]
- Coussi-Korbel S, Fragaszy DM. On the relation between social dynamics and social learning. Animal Behaviour. 1995;50:1441–1453. [Google Scholar]
- Dall SRX, Giraldeau LA, Olsson O, McNamara JM, Stephens DW. Information and its use by animals in evolutionary ecology. Trends in Ecology & Evolution. 2005;20:187–193. doi: 10.1016/j.tree.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Danchin E, Giraldeau LA, Valone TJ, Wagner RH. Public information: From nosy neighbors to cultural evolution. Science. 2004;305:487–491. doi: 10.1126/science.1098254. [DOI] [PubMed] [Google Scholar]
- Dean LG, Kendal RL, Schapiro SJ, Thierry B, Laland KN. Identification of the social and cognitive processes underlying human cumulative culture. Science. 2012;335:1114–1118. doi: 10.1126/science.1213969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapier M, Chauvin C, Thierry B. Tonkean macaques (Macaca tonkeana) find food sources from cues conveyed by group-mates. Animal Cognition. 2002;5:159–165. doi: 10.1007/s10071-002-0145-8. [DOI] [PubMed] [Google Scholar]
- Emery Thompson M, Muller MN, Kahlenberg SM, Wrangham RW. Dynamics of social and energetic stress in wild female chimpanzees. Hormones and Behavior. 2010;58:440–449. doi: 10.1016/j.yhbeh.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn E, Whiten A. Cultural transmission of tool use in young children: A diffusion chain study. Social Development. 2008;17:699–718. [Google Scholar]
- Fraser CP, Ruxton GD, Broom M. Public information and patch estimation for group foragers: A re-evaluation of patch-quitting strategies in a patchy environment. Oikos. 2006;112:311–321. [Google Scholar]
- Galef BG, Giraldeau LA. Social influences on foraging in vertebrates: Causal mechanisms and adaptive functions. Animal Behaviour. 2001;61:3–15. doi: 10.1006/anbe.2000.1557. [DOI] [PubMed] [Google Scholar]
- Giraldeau LA, Valone TJ, Templeton JJ. Potential disadvantages of using socially acquired information. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2002;357:1559–1566. doi: 10.1098/rstb.2002.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann E, Call J, Hernández-Lloreda MV, Hare B, Tomasello M. Humans have evolved specialized skills of social cognition: The cultural intelligence hypothesis. Science. 2007;317:1360–1366. doi: 10.1126/science.1146282. [DOI] [PubMed] [Google Scholar]
- Heyes CM. Social learning in animals: Categories and mechanisms. Biological Reviews. 1994;69:207–231. doi: 10.1111/j.1469-185x.1994.tb01506.x. [DOI] [PubMed] [Google Scholar]
- Hopper LM, Lambeth SP, Schapiro SJ. An evaluation of the efficacy of video displays for use with chimpanzees (Pan troglodytes) American Journal of Primatology. 2012;74:442–449. doi: 10.1002/ajp.22001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper LM, Lambeth SP, Schapiro SJ, Whiten A. Observational learning in chimpanzees and children studied through ‘ghost’ conditions. Proceedings of the Royal Society of London: Series B. Biological Sciences. 2008;275:835–840. doi: 10.1098/rspb.2007.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper LM, Schapiro SJ, Lambeth SP, Brosnan SF. Chimpanzees’ socially maintained food preferences indicate both conservatism and conformity. Animal Behaviour. 2011;81:1195–1202. doi: 10.1016/j.anbehav.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper LM, Spiteri A, Lambeth SP, Schapiro SJ, Horner V, Whiten A. Experimental studies of traditions and underlying transmission processes in chimpanzees. Animal Behaviour. 2007;73:1021–1032. [Google Scholar]
- Horner V, Proctor D, Bonnie KE, Whiten A, de Waal FBM. Prestige affects cultural learning in chimpanzees. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0010625. Online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner V, Whiten A. Causal knowledge and imitation/emulation switching in chimpanzees (Pan troglodytes) and children (Homo sapiens) Animal Cognition. 2005;8:164–181. doi: 10.1007/s10071-004-0239-6. [DOI] [PubMed] [Google Scholar]
- Horner V, Whiten A, Flynn E, de Waal FBM. Faithful replication of foraging techniques along cultural transmission chains by chimpanzees and children. PNAS: Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13878–13883. doi: 10.1073/pnas.0606015103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura S, Agnetta B, Hare B, Tomasello M. Chimpanzee use of human and conspecific social cues to locate hidden food. Developmental Science. 1999;2:448–456. [Google Scholar]
- Kendal JR, Rendell L, Pike TW, Laland KN. Nine-spined sticklebacks deploy a hill-climbing social learning strategy. Behavioral Ecology. 2009;20:238–244. [Google Scholar]
- Kendal R, Coolen I, Laland K. Adaptive trade-offs in the use of social and personal Information. In: Dukas R, Ratcliffe JM, editors. Cognitive Ecology II. Chicago, IL: The University of Chicago Press; 2009. pp. 249–271. [Google Scholar]
- Kendal RL, Coolen I, van Bergen Y, Laland KN. Trade-offs in the adaptive use of social and asocial learning. In: Slater PJB, Snowdon CT, Roper TJ, Brockmann H, Naguib M, editors. Advances in the Study of Behavior. Vol. 35. Waltham, MA: Academic Press; 2005. pp. 333–379. [Google Scholar]
- Kendal RL, Hopper LM, Brosnan SF, Schapiro SJ, Lambeth SP, Hoppitt W. Evidence for copying dominants and experts, but not the majority, in chimpanzees. 2013 Manuscript submitted for publication. [Google Scholar]
- Laidre ME. Eavesdropping foragers use level of collective commotion as public information to target high quality patches. Oikos. 2013 [Google Scholar]
- Laland KN. Social learning strategies. Learning & Behavior. 2004;32:4–14. doi: 10.3758/bf03196002. [DOI] [PubMed] [Google Scholar]
- Laland KN, Galef BG. The question of animal culture. Cambridge, MA: Harvard University Press; 2009. [Google Scholar]
- Lyons DE, Damrosch DH, Lin JK, Macris DM, Keil FC. The scope and limits of overimitation in the transmission of artefact culture. Philosophical Transactions of the Royal Society of London: Series B. Biological Sciences. 2011;366:1158–1167. doi: 10.1098/rstb.2010.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CF, Biro D, Matsuzawa T. Chimpanzees’ use of conspecific cues in matching-to-sample tasks: Public information use in a fully automated testing environment. Animal Cognition. 2011;14:893–902. doi: 10.1007/s10071-011-0424-3. [DOI] [PubMed] [Google Scholar]
- McQuoid LM, Galef BG. Social influences on feeding site selection by Burmese Fowl (Gallus-gallus) Journal of Comparative Psychology. 1992;106:137–141. [Google Scholar]
- Muller MN, Wrangham RW. Dominance, cortisol and stress in wild chimpanzees (Pan troglodytes schweinfurthii) Behavioral Ecology and Sociobiology. 2004;55:332–340. doi: 10.1007/s00265-020-02872-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CM, Eberly LE, Pusey AE. Foraging strategies as a function of season and rank among wild female chimpanzees (Pan troglodytes) Behavioral Ecology. 2006;17:1020–1028. [Google Scholar]
- Murray CM, Mane SV, Pusey AE. Dominance rank influences female space use in wild chimpanzees, Pan troglodytes: Towards an ideal despotic distribution. Animal Behaviour. 2007;74:1795–1804. [Google Scholar]
- Noser R, Byrne RW. How do wild baboons (Papio ursinus) plan their routes? Travel among multiple high-quality food sources with inter-group competition. Animal Cognition. 2010;13:145–155. doi: 10.1007/s10071-009-0254-8. [DOI] [PubMed] [Google Scholar]
- Orlet Fisher J, Mitchell DC, Wright HS, Birch LL. Parental influences on young girls’ fruit and vegetable, micronutrient, and fat intakes. Journal of the American Dietetic Association. 2002;102:58–64. doi: 10.1016/s0002-8223(02)90017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. Social traditions and social learning in capuchin monkeys (Cebus) Philosophical Transactions of the Royal Society of London: Series B. Biological Sciences. 2011;366:988–996. doi: 10.1098/rstb.2010.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader SM, Biro D. Experimental identification of social learning in wild animals. Learning & Behavior. 2010;38:265–283. doi: 10.3758/LB.38.3.265. [DOI] [PubMed] [Google Scholar]
- Reader SM, Laland KN. Social intelligence, innovation, and enhanced brain size in primates. Proceedings of the National Academy of Sciences, USA of the United States of America. 2002;99:4436–4441. doi: 10.1073/pnas.062041299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendell L, Fogarty L, Hoppitt WJE, Morgan TJH, Webster MM, Laland KN. Cognitive culture: Theoretical and empirical insights into social learning strategies. Trends in Cognitive Sciences. 2011;15(2):68–76. doi: 10.1016/j.tics.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Salvy SJ, Romero N, Paluch R, Epstein LH. Peer influence on pre-adolescent girls’ snack intake: Effects of weight status. Appetite. 2007;49:177–182. doi: 10.1016/j.appet.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Cortisol concentrations and the social significance of rank instability among wild baboons. Psychoneuroendocrinology. 1992;17:701–709. doi: 10.1016/0306-4530(92)90029-7. [DOI] [PubMed] [Google Scholar]
- Sayers K, Menzel CR. Memory and foraging theory: Chimpanzee utilization of optimality heuristics in the rank-order recovery of hidden foods. Animal Behaviour. 2012;84:795–803. doi: 10.1016/j.anbehav.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlag KH. Why imitate, and if so, how? A boundedly rational approach to multi-armed bandits. Journal of Economic Theory. 1998;78:130–156. [Google Scholar]
- Schlag KH. Which one should I imitate? Journal of Mathematical Economics. 1999;31:493–522. [Google Scholar]
- Slocombe KE, Zuberbühler K. Functionally Referential Communication in a Chimpanzee. Current Biology. 2005;15:1779–1784. doi: 10.1016/j.cub.2005.08.068. [DOI] [PubMed] [Google Scholar]
- Slocombe KE, Zuberbühler K. Food-associated calls in chimpanzees: Responses to food types or food preferences? Animal Behaviour. 2006;72:989–999. [Google Scholar]
- Templeton JJ, Giraldeau LA. Patch assessment in foraging flocks of european starlings - evidence for the use of public information. Behavioral Ecology. 1995;6:65–72. [Google Scholar]
- Tennie C, Call J, Tomasello M. Push or pull: Imitation vs. emulation in great apes and human children. Ethology. 2006;112:1159–1169. [Google Scholar]
- Tennie C, Call J, Tomasello M. Ratcheting up the ratchet: On the evolution of cumulative culture. Philosophical Transactions of the Royal Society of London: Series B. Biological Sciences. 2009;364:2405–2415. doi: 10.1098/rstb.2009.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennie C, Call J, Tomasello M. Untrained chimpanzees (Pan troglodytes schweinfurthii) fail to imitate novel actions. PLoS ONE. 2012;7:e41548. doi: 10.1371/journal.pone.0041548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valone TJ. Group foraging, public information, and patch estimation. Oikos. 1989;56:357–363. [Google Scholar]
- Valone TJ. From eavesdropping on performance to copying the behavior of others: A review of public information use. Behavioral Ecology and Sociobiology. 2007;62:1–14. [Google Scholar]
- Valone TJ, Templeton JJ. Public information for the assessment of quality: A widespread social phenomenon. Philosophical Transactions of the Royal Society of London: Series B. Biological Sciences. 2002;357:1549–1557. doi: 10.1098/rstb.2002.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schaik CP, Ancrenaz M, Borgen G, Galdikas B, Knott CD, Singleton I. Orangutan cultures and the evolution of material culture. Science. 2003;299:102–105. doi: 10.1126/science.1078004. [DOI] [PubMed] [Google Scholar]
- Visalberghi E, Addessi E. Seeing group members eating a familiar food enhances the acceptance of novel foods in capuchin monkeys. Animal Behaviour. 2000;60:69–76. doi: 10.1006/anbe.2000.1425. [DOI] [PubMed] [Google Scholar]
- Voelkl B, Huber L. Common marmosets (Callithrix jacchus) do not utilize social information in three simultaneous social foraging tasks. Animal Cognition. 2007;10:149–158. doi: 10.1007/s10071-006-0053-4. [DOI] [PubMed] [Google Scholar]
- Watts DP, Potts KB, Lwanga JS, Mitani JC. Diet of chimpanzees (Pan troglodytes schweinfurthii) at Ngogo, Kibale National Park, Uganda. Diet composition and diversity. American Journal of Primatology. 2012;74:114–129. doi: 10.1002/ajp.21016. [DOI] [PubMed] [Google Scholar]
- Webster MM, Laland KN. The learning mechanism underlying public information use in ninespine sticklebacks (Pungitius pungitius) Journal of Comparative Psychology. 2012 doi: 10.1037/a0029602. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Whiten A, Goodall J, McGrew WC, Nishida T, Reynolds V, Sugiyama Y. Cultures in chimpanzees. Nature. 1999;399:682–685. doi: 10.1038/21415. [DOI] [PubMed] [Google Scholar]
- Whiten A, Horner V, de Waal FBM. Conformity to cultural norms of tool use in chimpanzees. Nature. 2005;437:737–740. doi: 10.1038/nature04047. [DOI] [PubMed] [Google Scholar]
- Whiten A, Spiteri A, Horner V, Bonnie KE, Lambeth SP, Schapiro SJ. Transmission of multiple traditions within and between chimpanzee groups. Current Biology. 2007;17:1038–1043. doi: 10.1016/j.cub.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Wood LA, Kendal RL, Flynn EG. Context-dependent model-based biases in cultural transmission: Children’s imitation is affected by model age over model knowledge state. Evolution and Human Behavior. 2012;33:387–394. [Google Scholar]