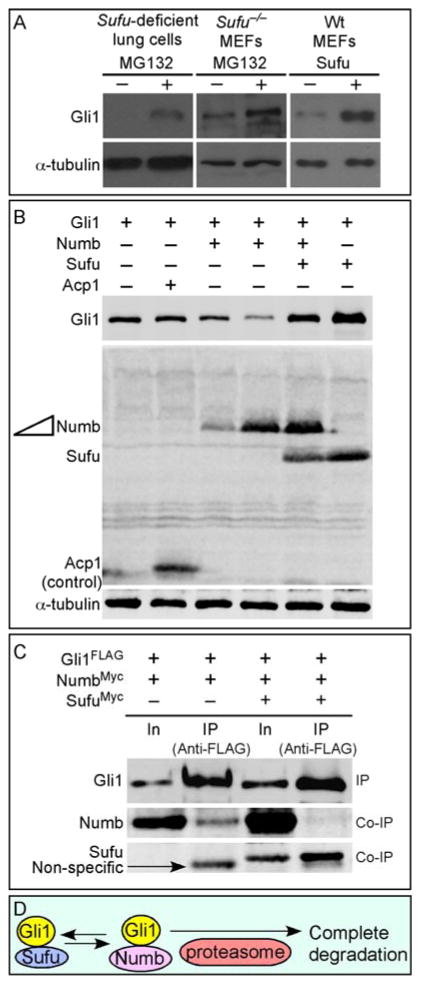
Figure 1. Control of Gli1 protein levels by Sufu.
(A) Western blot analysis of lysates from Sufu-deficient (Sufuf/−; Dermo1Cre/+) lung cells or Sufu null (Sufu−/−) MEFs treated with MG132 to block proteasome-mediated degradation. Endogenous Gli1 protein levels were elevated when protein degradation was inhibited in Sufu mutants. Similarly, protein levels of transfected Gli1 were increased when Sufu was co-expressed in wild-type (wt) MEFs. These results suggest that Sufu stabilizes Gli1 by preventing proteasome-dependent Gli1 degradation. Note that cycloheximide was added to block new protein synthesis in these studies. (B) Western blot analysis of lysates from HEK293T cells expressing various combinations of Gli1, Numb, Sufu and Acp1 (control). Numb expression resulted in reduction in Gli1 protein levels. This is consistent with previous reports in which Numb was shown to activate the E3 ligase Itch, leading to Gli1 ubiquitination and degradation. Numb-induced Gli1 reduction was reversed when Sufu was co-expressed with Numb. Tubulin was used as the loading control. (C) Western blot analysis of immunoprecipitated Gli1FLAG from HEK293T cell lysates to test the competition between Sufu and Numb in binding to Gli1. Co-immunoprecipitated NumbMyc by Gli1 was significantly reduced when SufuMyc was also pulled down by Gli1. (D) A model in which Sufu stabilizes Gli1 by inhibiting Numb-mediated protein degradation. In, input; IP, immunoprecipitation.