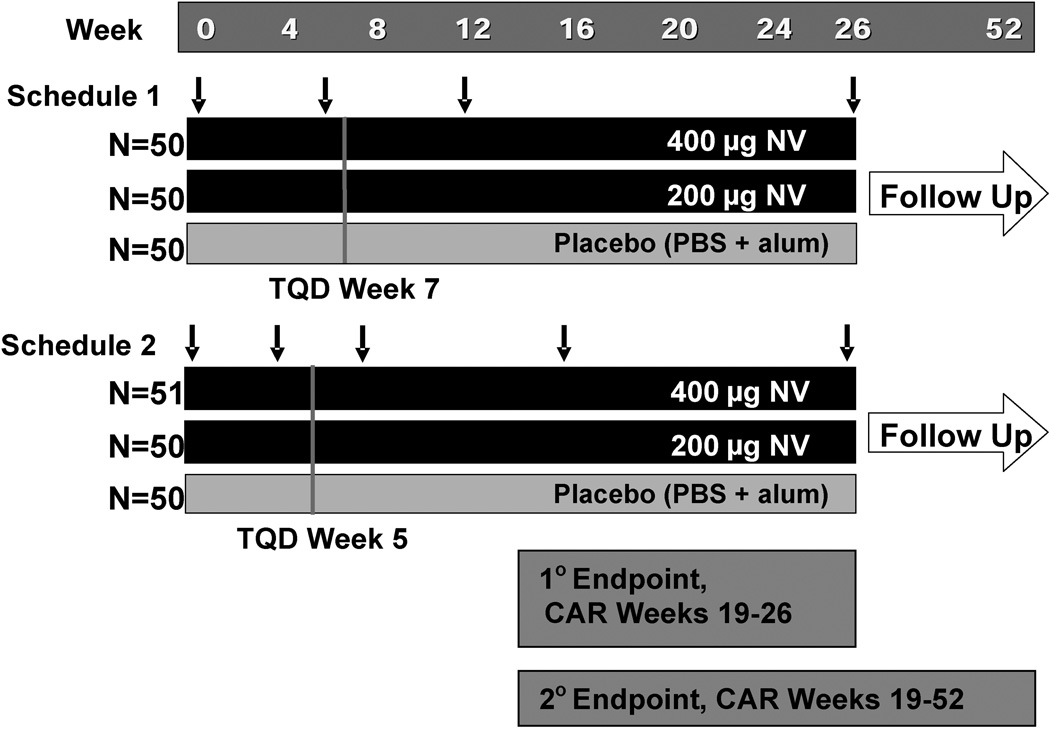
Figure 1.
Study design. Arrows denote timing of vaccinations for Schedule 1 (Week 0, 6, 12 and 26) and Schedule 2 (Week 0, 4, 8, 16, and 26). Primary endpoint (percent of subjects abstinent Week 19–26) and secondary endpoint (percent of subjects abstinent Week 19–52 are shown. NV (3’AmNic-rEPA); PBS (Phosphate buffered saline); Alum (aluminum hydroxide adjuvant); TQD (Target quit date).