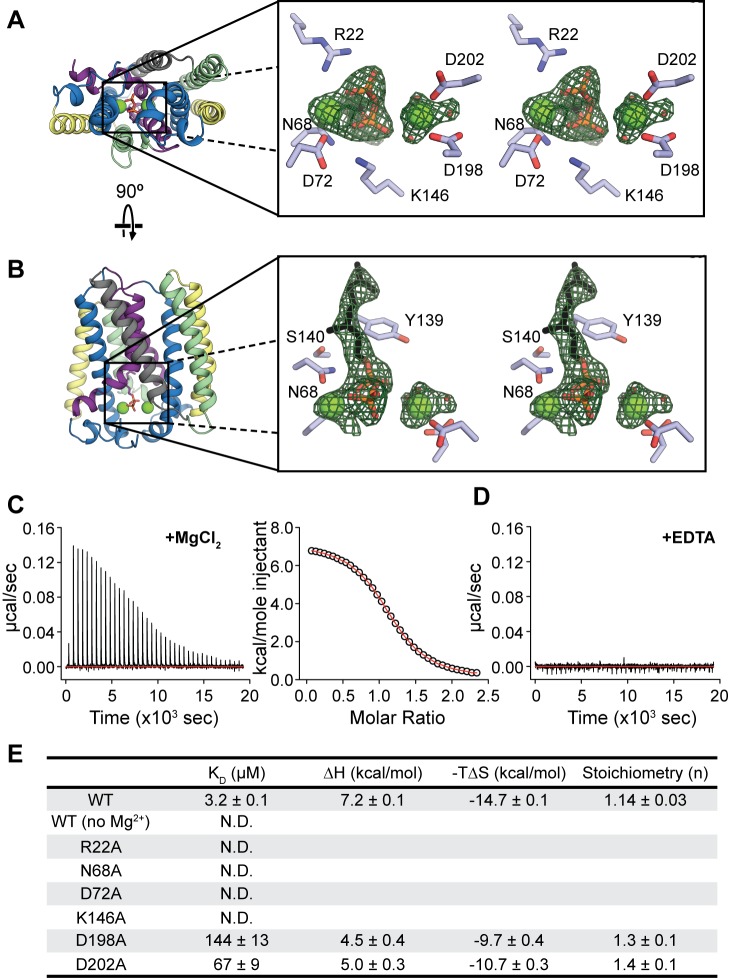
Figure 3. Substrate binding and active site.
(A) Stereo view of the GPP binding site, viewed from the cytoplasmic side of the membrane. Two Mg2+ atoms (green spheres) and a GPP molecule are shown in the binding site. Residues that potentially bind to Mg2+ and the diphosphate are labeled. (B) Stereo view of the active site from within the plane of the membrane. Conserved residues proposed to stabilize the intermediate state are labeled. The green mesh in both figures corresponds to Fo-Fc density contoured at 3.0 σ. (C and D) Binding of GPP to detergent-solubilized AfUbiA measured by ITC. Heats from successive injections of GPP were measured in the presence of 2 mM MgCl2 (C) or 1 mM EDTA (D). Right panel in (C) shows the fit to a one-site model. (E) Table of thermodynamic values for GPP binding to WT and mutant AfUbiA measured by ITC. KD, ΔH, and n were obtained by fitting a binding isotherm described in the Methods section. The thermodynamic relation ΔG = ΔH−TΔS was used to calculate −TΔS at 25°C with errors propagated. “N.D.” indicates no binding detected. Each value is the mean and s.e.m. of three ITC experiments.