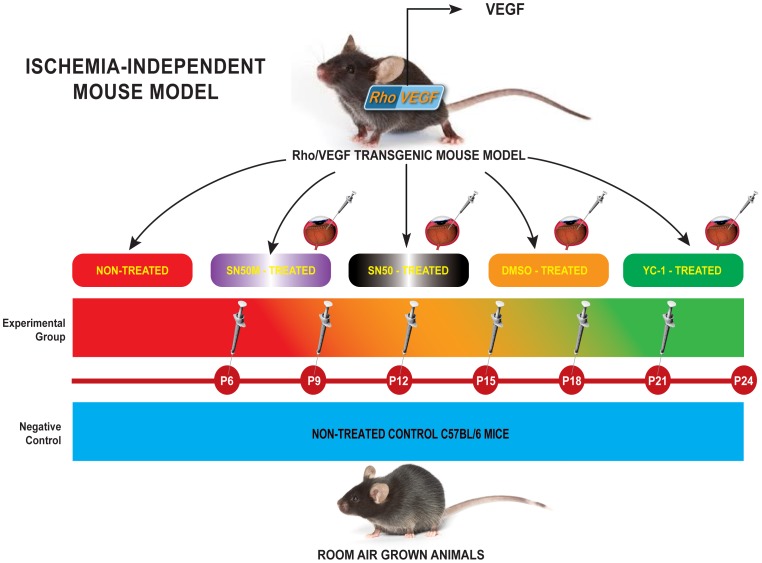
Figure 1. Experimental Approach of Targeting Retinal Vasculature in rho/VEGF Mouse Model.
C57BL/6 mice, which have been grown under ambient conditions, were used as controls. Whereas hemizygous rho/VEGF (line V6) transgenic mice in a C57BL/6 background, were used as the experimental animal model. Ocular NV was developed in rho/VEGF animals through the presence of the rhodopsin promoter, which drove the expression of VEGF in the photoreceptors. Mice were divided into four separate groups: Group I (Negative Control Group): Non-treated C57BL/6 mice (n = 15); Group II (Positive Control Group): Non-treated Rho/VEGF (n = 15); Group III (Mock-Treated Group): DMSO-treated Rho/VEGF mice (n = 15) — Mice received a sextuple regimen of intravitreous injections of either DMSO (0.2%) at P6, P9, P12, P15, P18, and P21; and Group IV (Drug-Treated Group): YC-1-treated Rho/VEGF mice (n = 15) — Mice received a sextuple regimen of intravitreous injections of YC-1 (100 µM) at P6, P9, P12, P15, P18, and P21]. At P24, the extent of subretinal NV was measured. For selective experiments that required the quantification of NFκB DNA binding activity and/or the measurements of NFκB expression at the message and the protein levels, two additional groups of mice were added to the experimental design; Group V (SN50-Treated Group): SN50-treated Rho/VEGF mice (n = 15) — Mice received a sextuple regimen of intravitreous injections of the NFκB competitive inhibitor peptide SN50 (20 µM) at P6, P9, P12, P15, P18, and P21. Whereas, Group VI (SN50M-Treated Group) —SN50M-treated Rho/VEGF mice (n = 15), received a sextuple regimen of intravitreous injections of the mutant control SN50M [20 µM] at P6, P9, P12, P15, P18, and P21.