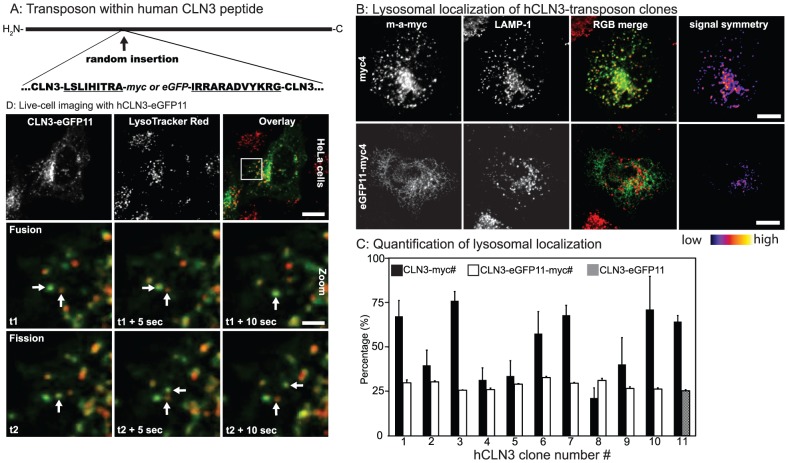
Figure 1. Generation of transposed hCLN3 clones.
A: The transposon inserted within the human CLN3 coding sequence produces an hCLN3 protein with a randomly inserted eGFP flanked by 9- and 12- amino acid isolating peptides (underlined). See Table 1 for transposon insertion sites. eGFP in hCLN3-eGFP clones was later exchanged for a myc-tag while retaining the isolating peptide sequences. B: HeLa cells transfected with hCLN3-myc or –eGFP11-myc clones were counterstained for myc-tag and lysosomal membrane protein LAMP-1, and visualized with confocal microscopy, and the level of myc-tag colocalizing with LAMP-1 was analyzed using an intensity-independent colocalization algorithm (see Mat&Met). The image in the right-most column reflects the intensity symmetry between the myc-tag and LAMP-1. Scale bars 10 µm C: The level of expressed hCLN3 clones in the LAMP-1 positive lysosomes was quantified. Addition of an eGFP to the hCLN3-myc clones reduced the level of their lysosomal localization. Note that the last column for chimeras in C shows the colocalization degree of hCLN3-eGFP11 without myc-tag N = 2, n>3. D: HeLa cells expressing hCLN3-eGFP11 with the most C-terminal eGFP were counter-stained with LysoTracker Red vital dye (LTR), and live-imaged with confocal microscope at 0.2 Hz frequency. Shown is a crop from a 5 minutes image sequence illustrating the partial lysosomal localization of hCLN3-eGFP11 (top row). Regularly, hCLN3-eGFP11 positive vesicles were seen to fuse (arrows in middle row) or dissociate (arrows in bottom row) from LTR positive structures. Scale bars 10 and 2 µm, respectively.