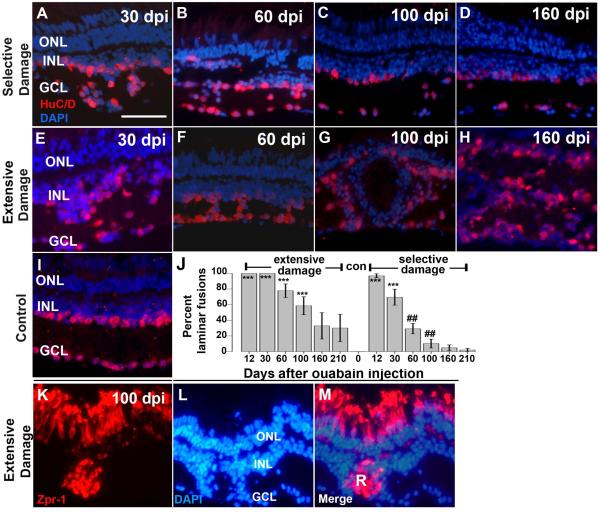
Figure 4.
Retinal lamination in regenerating retinas after selective or extensive retinal damage. A–I. Fixed, frozen retinal sections were immunolabeled with anti- HuC/D monoclonal antibody, to detect differentiated amacrine and ganglion cells, and mounted in the presence of the DAPI nuclear stain. Retinal lamination of regenerating retina after selective damage (A–D) and extensive damage (E–H), was assessed at 30 dpi (days post-injection; A,E), 60 dpi (B,F), 100dpi (C,G) and 160 dpi (D,H); examples of laminar fusions are indicated by white asterisks (*). Control section from a wild type retina is shown in (I). J. Barplot shows proportion of retinal sections showing laminar fusions (see Methods) following extensive or selective retinal damage as a function of recovery time; con=control (no laminar fusions). Asterisks (*) indicate significant differences from control (**, p<0.01; ***, p<0.001; two-sample Wilcoxon rank sum test) and the number signs (##) indicate significant differences between extensive and selective damage at the same recovery time (p<0.01; two-sample Wilcoxon rank sum test). K–M. Rosette formation in regenerating retina after extensive retinal damage. Fixed, frozen retinal sections were immunolabeled with the zpr-1 monoclonal antibody (K), to detect differentiated cone photoreceptors, and mounted in the presence of the DAPI nuclear stain (L; merged images shown in M). Photoreceptor rosettes (R) are present in retina 100 dpi (days post-injection). This experiment was carried out for selectively damaged retina, but rosettes were rarely observed (Table 2). Scale bar (applies to A-I and K-M) = 25 μm. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer).