Abstract
Strong epidemiological evidence suggests that smokers and coffee drinkers have a lower risk of Parkinson’s disease (PD). The explanation for this finding is still unknown and the discussion has focused on two main hypotheses. The first suggests that PD patients have premorbid personality traits associated with dislike for coffee-drinking and smoking. The second posits that caffeine and nicotine are neuroprotective. We propose an alternative third hypothesis in which both cigarette and coffee consumption change the composition of the microbiota in the gut in a way that mitigates intestinal inflammation. This, in turn, would lead to less misfolding of the protein alpha-synuclein in enteric nerves, reducing the risk of PD by minimizing propagation of the protein aggregates to the central nervous system where they otherwise can induce neurodegeneration.
Background
A striking epidemiological feature of Parkinson's disease (PD) is that it is less common among cigarette smokers and coffee drinkers. The first case-control study suggesting such an inverse association was published more than 40 years ago. The finding has, since then, been confirmed by several other surveys.1 In their systematic review and meta-analysis of 61 case-control and cohort studies published on the topic in 2001, Hernan and colleagues showed that the risk of PD is 60% lower among current cigarette smokers than among never smokers, and 30% lower among coffee drinkers than among non–drinkers.2 These data, mainly obtained from retrospective studies were confirmed in four large prospective studies for either coffee and cigarette consumption (reviewed in 3). A recent study has shown that the consumption of caffeine-containing beverages other than coffee, such as black tea and Japanese and Chinese teas, is also inversely related to PD risk.4
Although the high number of studies conducted and the magnitude of the observed associations provide unequivocal epidemiological evidence that the risk of PD is lower in smokers and coffee drinkers, the explanations for these findings still remain controversial.3 Several small case-control studies have suggested that PD patients display specific personality traits such as cautiousness, high harm avoidance and lack of novelty seeking even before the onset of motor symptoms. This has lead to the suggestion that people who later develop PD are constitutionally less likely to feel the need for the type of stimulation provided by tobacco and coffee.5 Another possible explanation comes from the observations that substances present in coffee and tobacco, among which caffeine and nicotine are the most obvious candidates, are neuroprotective in experimental settings. Indeed, both nicotine and caffeine have, for example, been found to reduce MPTP-induced neurotoxicity in animal models of PD.6,7 Albeit plausible, these two hypotheses are still contested. The idea that PD patients exhibit a premorbid personality has never been explored in a large prospective study8 and there are obvious and well-known limitations inherent in animal models of PD.9
The hypothesis
Is there an alternative explanation to the inverse association between the risk of PD and coffee and cigarette consumption? We think so and we suggest that it lies hidden in digestive tract and more precisely in the gut microbiota. The intestinal microbiota consists of around a hundred trillion microorganisms that reside primarily in the lower gastro-intestinal tract, largely outnumbering our eukaryotic cells and surpassing the metabolic potential of our body.10 Interestingly, the influence of microbiota is not limited to local effects but also extends to remote organs, particularly the brain.11,12 Several recent studies have tried to shed light on the influence of gut flora on the brain by using germ-free animals or disrupting existing microbiota, and exposing the animals to specific microorganisms.11 Although the precise mechanisms through which signals from gut bacteria are communicated to the brain are still largely unknown, evidence obtained from vagotomy experiments point toward a key role for the vagus nerve in the interplay between the microbiota and the brain.11
In healthy subjects, the intestinal microbiota is generally stable over time, but compositional changes might occur following antibiotic usage or dietary modifications.10 Diseases associated with impaired gastrointestinal motility, such as diabetes mellitus and PD, predispose for small intestinal bacterial overgrowth (SIBO), a malabsorption syndrome associated with increased bacterial density in the gut.13,14 Interestingly, three reports recently addressed the role of smoking and coffee on the composition of gut microbiota. A marked shift in the composition of the intestinal microbiota was observed in humans after smoking cessation15 while consumption of coffee in both mice and humans induced a significant increase in the Bifidobacterium population without major impact on the dominant microbes.16,17 We postulate that the changes in gut bacteria observed after coffee consumption and cigarette smoking affect the risk of PD being triggered in the gastrointestinal tract.
In their comprehensive anatomopathological survey published in 2003, Braak and coworkers postulated that α-synuclein-positive Lewy pathology initially appears, during the pre-motor stage of PD, in the dorsal motor nucleus of the vagus.18 Subsequent work from the same group along with two studies using gastrointestinal biopsies19,20 suggested that the enteric neurons, which synapse with both afferent and efferent vagal neurons, are also affected by Lewy pathology early in the disease.21 These and other observations led Braak to put forth the so-called "dual hit hypothesis" stipulating that PD may be triggered by an hitherto unknown neurotropic agent, presumed to be a virus, which initially affects the gut and the olfactory system causing α-synuclein aggregation.22 Thereafter, according to the hypothesis, the neurotropic agent propagates through the nervous system, giving rise to Lewy pathology on the way and eventually after several years, reaching the substantia nigra. More recent observations showing in experimental models that misfolded α-synuclein can propagate from one neuron to another in a prion-like fashion has led to the hypothesis that it is misfolded α-synuclein itself that is the propagating agent.23 Thus, the initial event would be α-synuclein misfolding and aggregation in neurons of the submucosal plexus whose terminal axons are only micrometers away from the gut lumen and flora.24,25 Several detailed pathological studies have indeed consistently shown that these nerve terminals were affected by Lewy path The pathological process would further spread to the CNS via the vagal preganglionic innervation of the gut24,26, as this has been already demonstrated for prion27 and neural tracers28.
If the beneficial effects of smoking and coffee consumption on PD are mediated through the modulation of the microbiota-gut-brain axis, what role might the microbes play in Braak’s scenario? A possible explanation comes from the anti-inflammatory properties of some bacterial stains such as Bifidobacterium whose activity and proportion are upregulated by coffee-drinking.17 One might therefore suggest that in the absence of coffee drinking and cigarette smoking, the microbiota would shift toward a pro-inflammatory state (Figure 1). This would promote chronic gastrointestinal inflammation and an enteric glial reaction, which actually have been shown to occur in the early stage of PD.29 The local inflammation would in turn initiate the neuropathological process by making α-synuclein more prone to aggregate within the adjacent submucosal neurons.30,31 As an alternative explanation, coffee and tobacco might promote microbes that counteract certain forms of chronic gastrointestinal infection such as that caused by Helicobacter Pylori, which is overrepresented in PD patients as compared to controls subjects.32
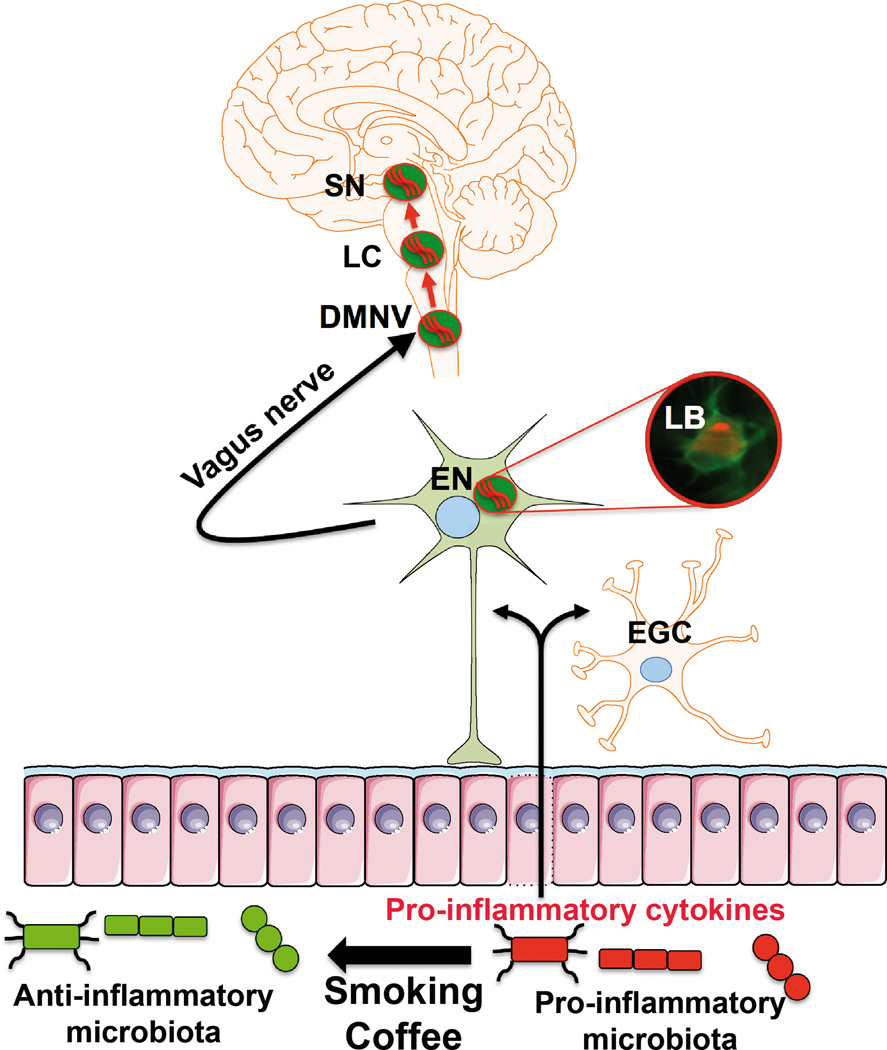
Figure 1. Possible role of smoking and coffee consumption on microbiota-gut-brain-axis and the development of Parkinson’s disease.
We propose that both cigarette smoking and coffee consumption may induce changes in the composition of microbiota with a shift toward a more anti-inflammatory state. In the absence of coffee and cigarette smoking, more pro-inflammatory cytokines are produced by immunologically competent cells and by enteric glial cells (EGC) in the gut. This would promote α-synuclein aggregation (Lewy bodies, LB) within enteric neurons (EN) that may spread further to the central nervous system via the vagal preganglionic innervation of the gut and the dorsal motor nucleus of the vagus (DMNV). After several years, the pathological process would reach the locus coeruleus (LC) and the substantia nigra (SN).
Besides the vagal neuronal pathway, a putative mechanism by which gut flora bacteria may influence the brain include bacterial products that gain access to the brain via the bloodstream and the area postrema12. Systemic inflammation has been demonstrated in PD patients33,34 and evidence from animal models supports a role for peripheral inflammation in the exacerbation of neurodegeneration.35 It could thus be proposed that smoking and coffee consumption, by decreasing the release of pro-inflammatory cytokines from the gut to the bloodstream, may reduce central nervous system neurodegeneration.
Testing the hypothesis
Complex studies would be required to determine whether a link exists between tobacco and coffee-drinking, changes in the gut microbe composition and a lower risk of PD. Our hypothesis suggests that in people who develop PD the crucial changes in the gut microbiome might occur several years before they present with motor symptoms. These changes might therefore no longer be apparent at the time of PD diagnosis, making it challenging to test the hypothesis. Consequently, the first critical and more feasible studies could explore if changes in the gut microbiome are capable of modifying the evolution profile in experimental parkinsonism. Disrupting the microbiome or adding probiotics in toxin-induced or genetic models of PD would provide interesting answers. It could be also relevant to compare the neurodegenerative changes induced by a neurotoxin, such as MPTP and rotenone, between germ-free and specific pathogen-free animals, an approach that successfully has revealed that gut microbiota is critically involved in the development of experimental autoimmune encephalomyelitis in mice.36 If a first set of experiments in animal models of PD is encouraging, further work will be needed to determine whether the specific changes in microbiota composition induced by coffee and tobacco are sufficient to prevent neurodegeneration in experimental parkinsonism and whether these effects are vagal-dependent. Combined with clinical studies on the gut microbiota in PD, the results of such studies in animal models of PD should help us find out if the truth lies in the gut.
Footnotes
Authors Roles
All authors initially discussed the presented concepts. Pascal Derkinderen wrote the first draft. All authors edited the manuscript.
Financial Disclosures of all authors related to this work (for the preceding 12 months)
Pascal Derkinderen has nothing to disclose. Kathleen Shannon has nothing to disclose. Patrik Brundin has nothing to disclose.
References
- 1.Nefzger MD, Quadfasel FA, Karl VC. A retrospective study of smoking in Parkinson's disease. Am. J. Epidemiol. 1968;88(2):149–158. doi: 10.1093/oxfordjournals.aje.a120874. [DOI] [PubMed] [Google Scholar]
- 2.Hernán MA, Takkouche B, Caamaño-Isorna F, Gestal-Otero JJ. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson's disease. Annals of neurology. 2002;52(3):276–284. doi: 10.1002/ana.10277. [DOI] [PubMed] [Google Scholar]
- 3.Wirdefeldt K, Adami H-O, Cole P, et al. Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur J Epidemiol. 2011;26(S1):1–58. doi: 10.1007/s10654-011-9581-6. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka K, Miyake Y, Fukushima W, et al. Intake of Japanese and Chinese teas reduces risk of Parkinson's disease. Parkinsonism & related disorders. 2011;17(6):446–450. doi: 10.1016/j.parkreldis.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Evans AH, Lawrence AD, Potts J, et al. Relationship between impulsive sensation seeking traits, smoking, alcohol and caffeine intake, and Parkinson's disease. Journal of neurology, neurosurgery, and psychiatry. 2006;77(3):317–321. doi: 10.1136/jnnp.2005.065417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maggio R, Riva M, Vaglini F, et al. Nicotine prevents experimental parkinsonism in rodents and induces striatal increase of neurotrophic factors. J Neurochem. 1998;71(6):2439–2446. doi: 10.1046/j.1471-4159.1998.71062439.x. [DOI] [PubMed] [Google Scholar]
- 7.Chen JF, Xu K, Petzer JP, et al. Neuroprotection by caffeine and A(2A) adenosine receptor inactivation in a model of Parkinson's disease. J Neurosci. 2001;21(10):RC143. doi: 10.1523/JNEUROSCI.21-10-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poletti M, Bonuccelli U. Personality traits in patients with Parkinson's disease: assessment and clinical implications. Journal of neurology. 2012;259(6):1029–1038. doi: 10.1007/s00415-011-6302-8. [DOI] [PubMed] [Google Scholar]
- 9.Fox SH, Brotchie JM. The MPTP-lesioned non-human primate models of Parkinson's disease. Past, present, and future. Progress in brain research. 2010;184:133–157. doi: 10.1016/S0079-6123(10)84007-5. [DOI] [PubMed] [Google Scholar]
- 10.Lozupone CA, Stombaugh JI, Gordon JI, et al. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forsythe P, Kunze WA, Bienenstock J. On communication between gut microbes and the brain. Current Opinion in Gastroenterology. 2012;28(6):557–562. doi: 10.1097/MOG.0b013e3283572ffa. [DOI] [PubMed] [Google Scholar]
- 12.Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nature Reviews Microbiology. 2012;10(11):735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 13.Gabrielli M, Bonazzi P, Scarpellini E, et al. Prevalence of small intestinal bacterial overgrowth in Parkinson's disease. Mov Disord. 2011;26(5):889–892. doi: 10.1002/mds.23566. [DOI] [PubMed] [Google Scholar]
- 14.Fasano A, Bove F, Gabrielli M, et al. The role of small intestinal bacterial overgrowth in Parkinson's disease. Mov Disord. 2013;28(9):1241–1249. doi: 10.1002/mds.25522. [DOI] [PubMed] [Google Scholar]
- 15.Biedermann L, Zeitz J, Mwinyi J, et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PLoS One. 2013;8(3):e59260. doi: 10.1371/journal.pone.0059260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakayama T, Oishi K. Influence of coffee (Coffea arabica) and galacto-oligosaccharide consumption on intestinal microbiota and the host responses. FEMS Microbiol. Lett. 2013;343(2):161–168. doi: 10.1111/1574-6968.12142. [DOI] [PubMed] [Google Scholar]
- 17.Jaquet M, Rochat I, Moulin J, et al. Impact of coffee consumption on the gut microbiota: a human volunteer study. Int. J. Food Microbiol. 2009;130(2):117–121. doi: 10.1016/j.ijfoodmicro.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Braak H, Del Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiology of aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 19.Shannon KM, Keshavarzian A, Dodiya HB, et al. Is alpha-synuclein in the colon a biomarker for premotor Parkinson's disease? Evidence from 3 cases. Mov Disord. 2012;27(6):716–719. doi: 10.1002/mds.25020. [DOI] [PubMed] [Google Scholar]
- 20.Hilton D, Stephens M, Kirk L, et al. Accumulation of α-synuclein in the bowel of patients in the pre-clinical phase of Parkinson's disease. Acta neuropathologica. 2013 doi: 10.1007/s00401-013-1214-6. [DOI] [PubMed] [Google Scholar]
- 21.Walter GC, Phillips RJ, Baronowsky EA, Powley TL. Versatile, high-resolution anterograde labeling of vagal efferent projections with dextran amines. Journal of neuroscience methods. 2009;178(1):1–9. doi: 10.1016/j.jneumeth.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawkes CH, Del Tredici K, Braak H. Parkinson's disease: a dual-hit hypothesis. Neuropathology and applied neurobiology. 2007;33:599–614. doi: 10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angot E, Steiner JA, Hansen C, et al. Are synucleinopathies prion-like disorders? Lancet neurology. 2010;9(11):1128–1138. doi: 10.1016/S1474-4422(10)70213-1. [DOI] [PubMed] [Google Scholar]
- 24.Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner's and Auerbach‘s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396(1):67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Cersosimo MG, Benarroch EE. Neural control of the gastrointestinal tract: implications for Parkinson disease. Mov Disord. 2008;23(8):1065–1075. doi: 10.1002/mds.22051. [DOI] [PubMed] [Google Scholar]
- 26.Phillips RJ, Walter GC, Wilder SL, et al. Alpha-synuclein-immunopositive myenteric neurons and vagal preganglionic terminals: autonomic pathway implicated in Parkinson's disease? Neuroscience. 2008;153(3):733–750. doi: 10.1016/j.neuroscience.2008.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McBride PA, Schulz-Schaeffer WJ, Donaldson M, et al. Early spread of scrapie from the gastrointestinal tract to the central nervous system involves autonomic fibers of the splanchnic and vagus nerves. Journal of virology. 2001;75(19):9320–9327. doi: 10.1128/JVI.75.19.9320-9327.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powley TL, Fox EA, Berthoud HR. Retrograde tracer technique for assessment of selective and total subdiaphragmatic vagotomies. The American journal of physiology. 1987;253(2 Pt 2):R361–R370. doi: 10.1152/ajpregu.1987.253.2.R361. [DOI] [PubMed] [Google Scholar]
- 29.Devos D, Lebouvier T, Lardeux B, et al. Colonic inflammation in Parkinson's disease. Neurobiol Dis. 2013;50:42–48. doi: 10.1016/j.nbd.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Pouclet H, Lebouvier T, Coron E, et al. A comparison between colonic submucosa and mucosa to detect Lewy pathology in Parkinson's disease. Neurogastroenterol Motil. 2012;24(4):e202–e205. doi: 10.1111/j.1365-2982.2012.01887.x. [DOI] [PubMed] [Google Scholar]
- 31.Lema Tomé CM, Tyson T, Rey NL, et al. Inflammation and α-synuclein“s prion-like behavior in Parkinson”s disease--is there a link? Mol. Neurobiol. 2013;47(2):561–574. doi: 10.1007/s12035-012-8267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nielsen HH, Qiu J, Friis S, et al. Treatment for Helicobacter pylori infection and risk of Parkinson's disease in Denmark. Eur J Neurol. 2012;19(6):864–869. doi: 10.1111/j.1468-1331.2011.03643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reale M, Iarlori C, Thomas A, et al. Peripheral cytokines profile in Parkinson's disease. Brain Behav Immun. 2009;23(1):55–63. doi: 10.1016/j.bbi.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Lindqvist D, Kaufman E, Brundin L, et al. Non-motor symptoms in patients with Parkinson's disease - correlations with inflammatory cytokines in serum. PLoS One. 2012;7(10):e47387. doi: 10.1371/journal.pone.0047387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernandez-Romero MC, Delgado-Cortes MJ, Sarmiento M, et al. Peripheral inflammation increases the deleterious effect of CNS inflammation on the nigrostriatal dopaminergic system. Neurotoxicology. 2012;33(3):347–360. doi: 10.1016/j.neuro.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 36.Berer K, Mues M, Koutrolos M, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479(7374):538–541. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]